Alpha-amylase
a technology of alpha-amylase and amylase, which is applied in the field of alpha-amylase, can solve the problems of granule splitting, affecting the volume, texture and appearance of loaf, and affecting the quality of loaf,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of the Alpha-Amylase of the Invention
Cloning and Enzyme Preparation
[0394]As described in further detail below the alpha-amylase gene was cloned and expressed in B. subtilis in the following way
Strains and Plasmids
[0395]Bacillus subtilis strain BS154 (CBS 363.94) (ΔaprE, ΔnprE, amyE-, spo-) is described in Quax and Broekhuizen 1994 Appl Microbiol Biotechnol. 41: 425-431.
[0396]The E. coli / B. subtilis shuttle vector pBHA12 is described in (WO2008 / 000632). Alicyclobacillus pohliae NCIMB14276 was described by Imperio et al (Int. J. Syst. Evol. Microbiol 58:221-225, 2008).
[0397]Bacillus stearothermophilus C599 (NCIMB11873) is described in WO91 / 04669.
Molecular Biology Techniques
[0398]Molecular biology techniques known to the skilled person were performed (see: Sambrook & Russell, Molecular Cloning: A Laboratory Manual, 3rd Ed., CSHL Press, Cold Spring Harbor, N.Y., 2001). Polymerase chain reaction (PCR) was performed on a thermocycler with Phusion High-Fidelity DNA polymerase (F...
example 2
Expression of A. pohliae DSM-AM Gene in Bacillus subtilis
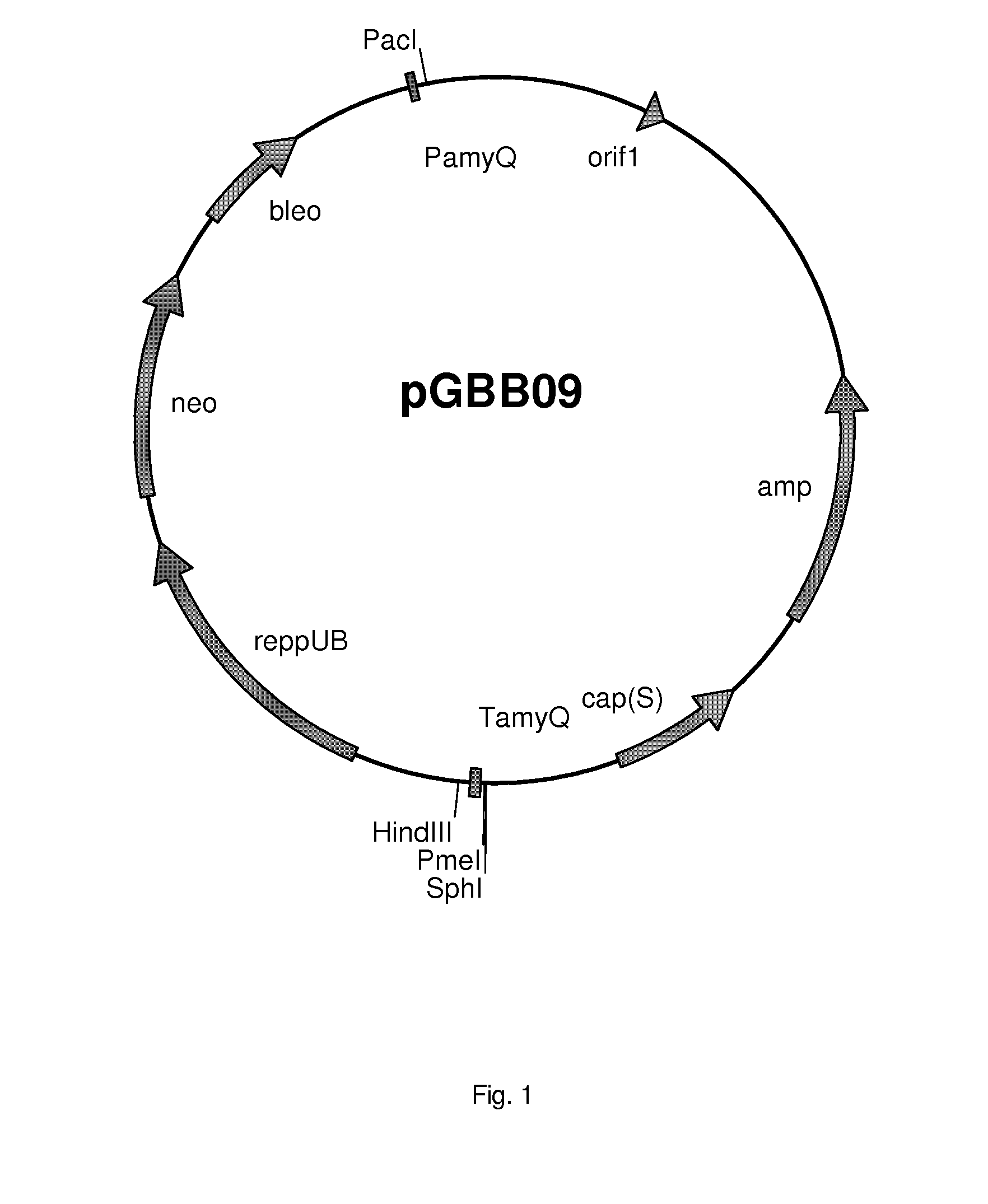
[0403]An amyQ terminator and a PmeI restriction site were introduced in the pBHA12 vector by digesting pBHA12 with SphI and HindIII and cloning the following DNA sequence 5′-GCATGCGTTTAAACAAAAACACCTCCAAGCTGAGTGCGGGTATCAGCTTGGAGGTGC GTTTATTTTTTCAGCCGTATGACAAGGTCGGCATCAGAAGCTT-3′ (the 5′SphI and 3′HindIII restriction sites are underlined).
[0404]The fragment was cloned into pBHA12 which resulted in vector pGBB09 (FIG. 1).
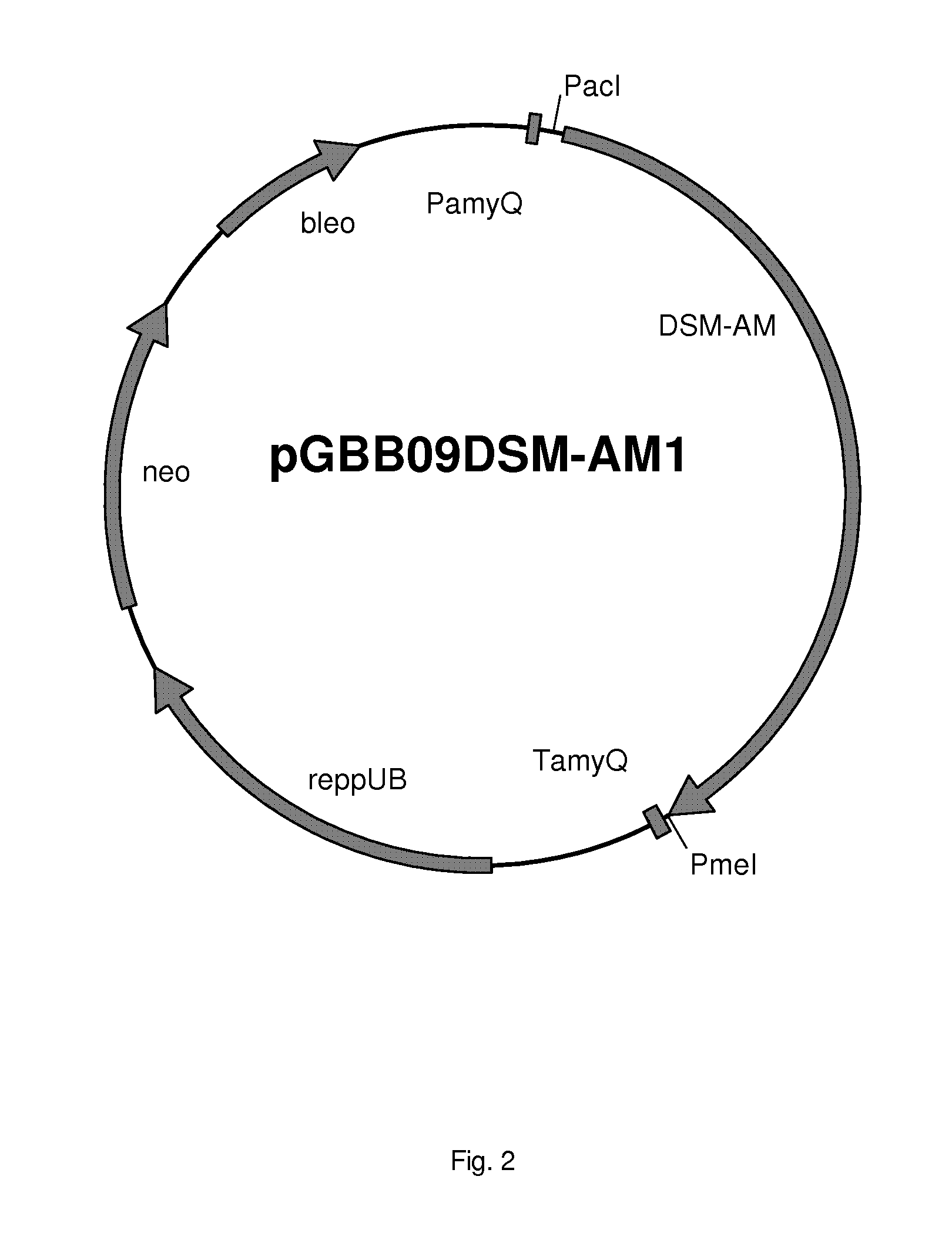
[0405]The DSM-AM gene was synthesised by GeneArt (Germany) and at the 5′ end the PacI restriction site was added and at its 3′end the PmeI restriction site was added. The DSM-AM gene was cloned into the Pad and PmeI digested pGBB09 vector which resulted in vector pGBB09DSM-AM1 (FIG. 2). This vector was transformed to B. subtilis strain BS154. The sequence of the plasmid was confirmed by DNA sequencing. The B. subtilis strain BS154 containing pGBB09DSM-AM1 was named DSM-AMB154-1.
example 3
Expression of DSM-AM with B. subtilis in Shake Flasks
[0406]B. subtilis strains DSM-AMB154-1 and BS154 were grown in a shake flask. These shake flasks contained 20 ml 2×TY medium composed of 1.6% (w / v) Bacto tryptone, 1% (w / v) Yeast extract and 0.5% (w / v) NaCl. The cultures were shaken vigorously at 37° C. and 250 rpm for 16 hours and 0.2 ml culture medium was used to inoculate 20 ml SMM medium. SMM pre-medium contains 1.25% (w / w) yeast extract, 0.05% (w / w) CaCl2, 0.075% (w / w) MgCl2.6H2O, 15 μg / l MnSO4.4H2O, 10 μg / l CoCl2.6H2O, 0.05% (w / w) citric acid, 0.025% (w / w) antifoam 86 / 013 (Basildon Chemicals, Abingdon, UK). To complete SMM medium, 20 ml of 5% (w / v) maltose and 20 ml of a 200 mM Na-phosphate buffer stock solution (pH 6.8), both prepared and sterilized separately, were added to 60 ml SMM pre-medium. These cultures were incubated for 48 hours at 37° C. and 250 rpm. The supernatants were harvested and analysed for enzyme productivity. The alpha-amylase activity of strain DSM-AMB...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com