Reducing electroless silver plating solution and reducing electroless silver plating method
a technology of electroless silver plating and which is applied in the direction of liquid/solution decomposition chemical coating, solid/suspension decomposition chemical coating, coating, etc., can solve the problems of limited underlying material used for substituting electroless silver plating, the inability to form plating films with good film characteristics, and the inability to reduce electroless silver plating solution. , to prevent the decomposition of silver, the effect of reducing the solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
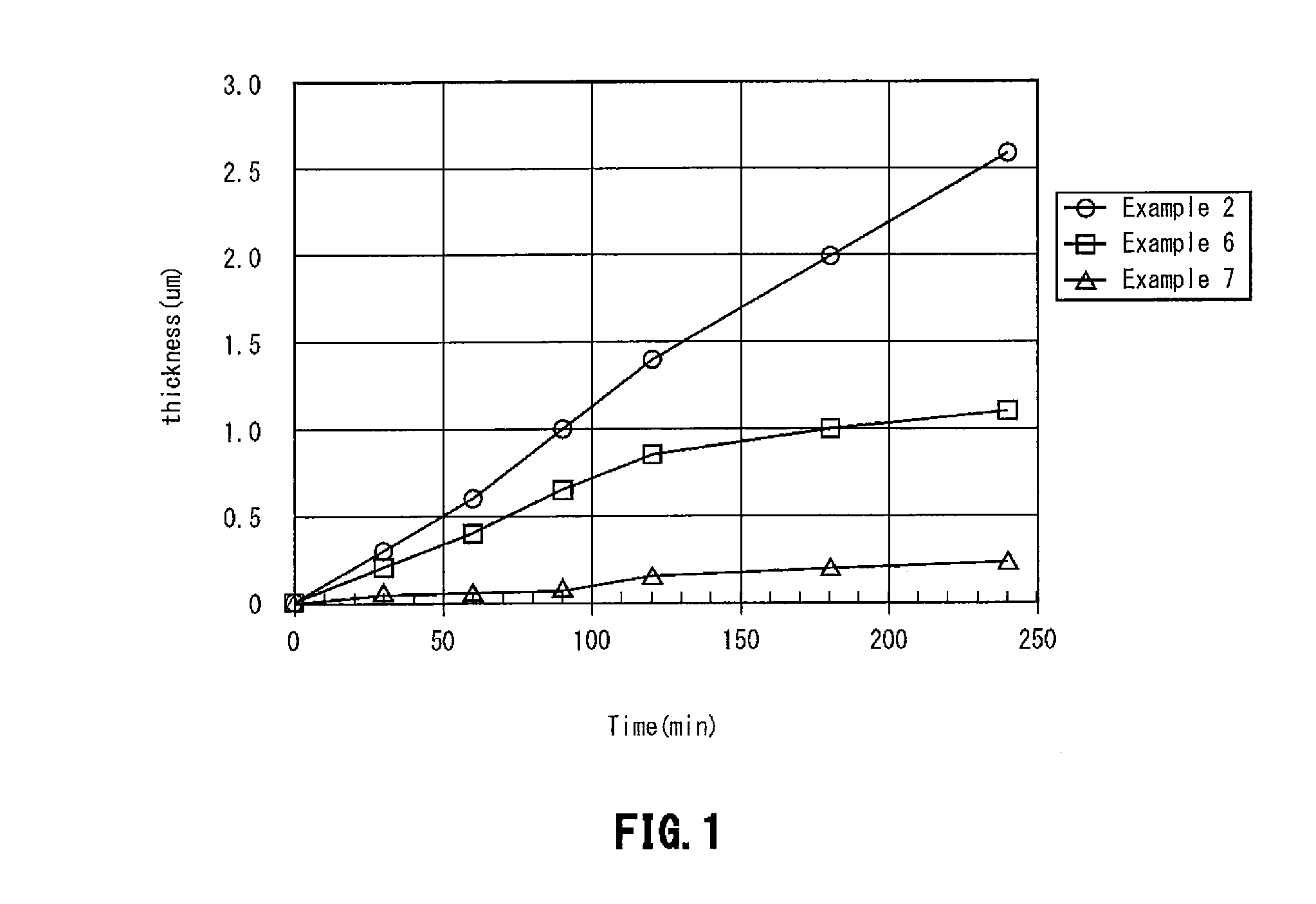
Image
Examples
example 1
[0057]There was prepared an aqueous solution containing a silver nitrate having a silver concentration of 9.0×10−3 mol / L (1.0 g / L), a hydroxylammonium salt (hydroxylammonium sulfate) in a concentration of 1.24×10−3 mol / L as a reducing agent, and EDTA in a concentration of 0.15 mol / L (50 g / L) as a complexing agent, and furthermore, potassium cyanide in a concentration of 1 mg / L was added thereto, whereby the aqueous solution is made to have a cyanide ion concentration of 0.006×10−3 mol / L in a plating solution, and the aqueous solution is adjusted using caustic soda to have a pH of 9.0, whereby a reducing electroless silver plating solution was prepared.
example 2
[0058]A reducing electroless silver plating solution was prepared in the same manner as in Example 1, except that potassium cyanide in a concentration of 300 mg / L was added, thereby allowing the plating solution to have a cyanide ion concentration of 1.8×10−3 mol / L.
example 3
[0059]A reducing electroless silver plating solution was prepared in the same manner as in Example 1, except that potassium cyanide in a concentration of 500 mg / L was added, thereby allowing the plating solution to have a cyanide ion concentration of 3.0×10−3 mol / L.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com