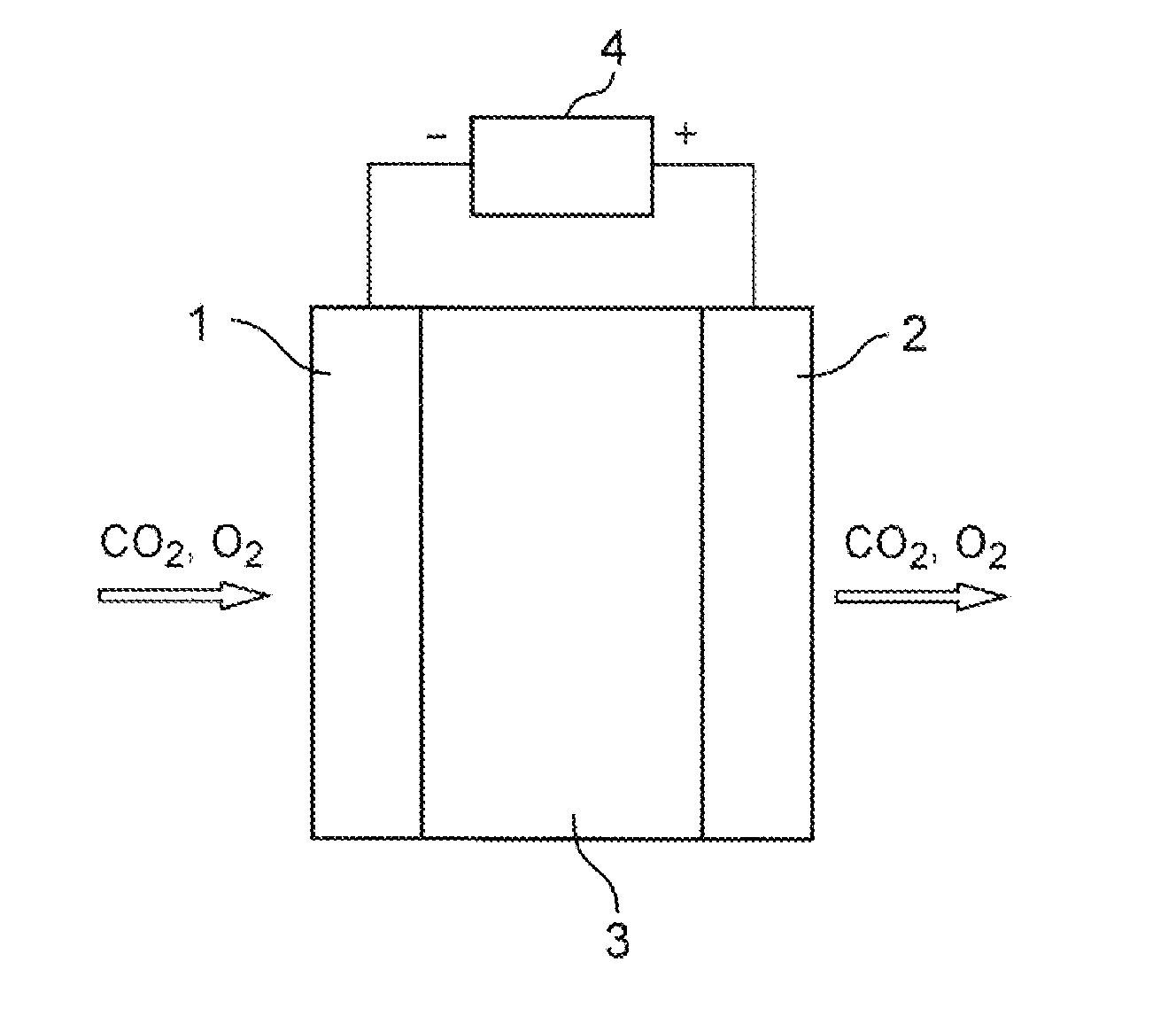
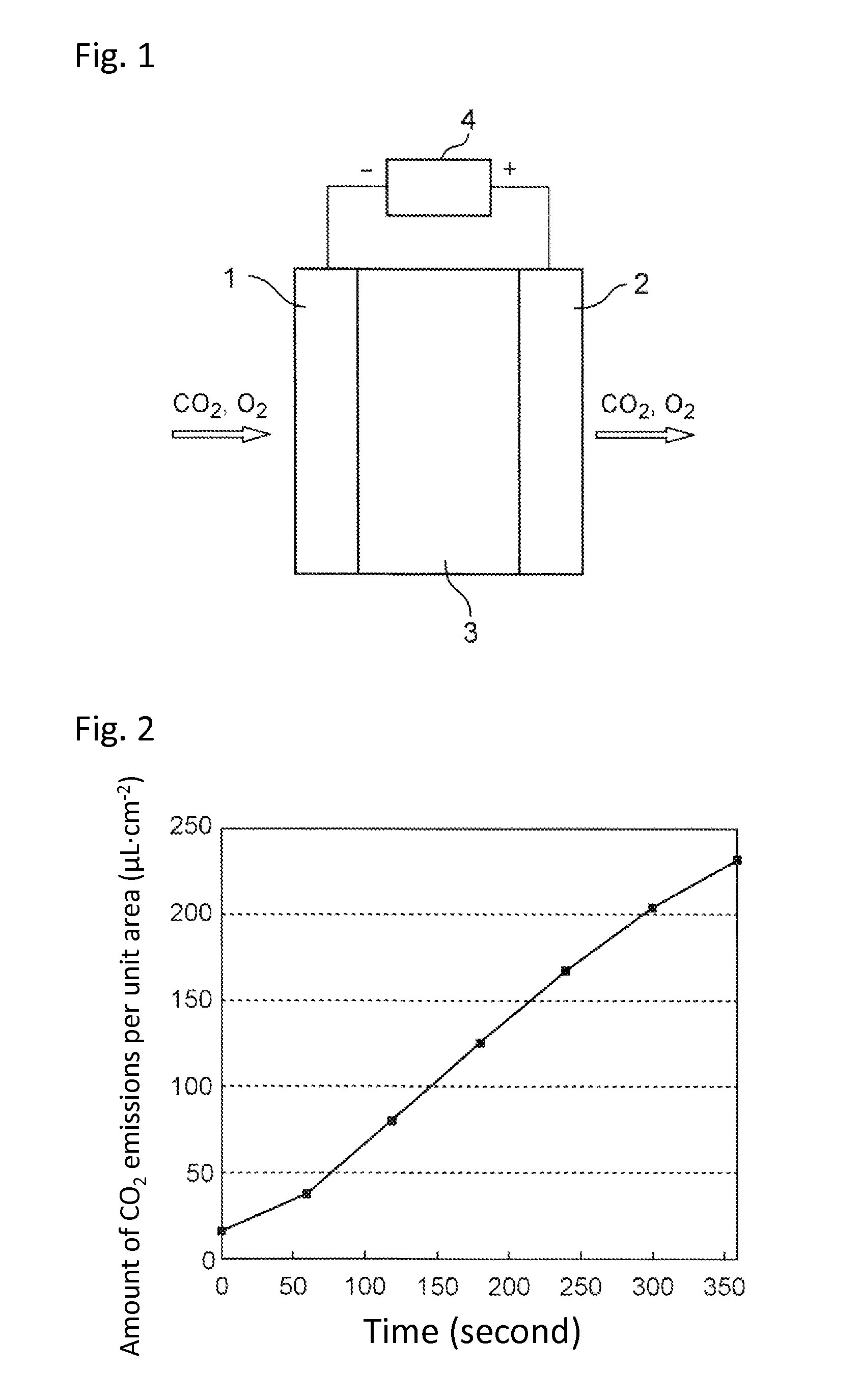
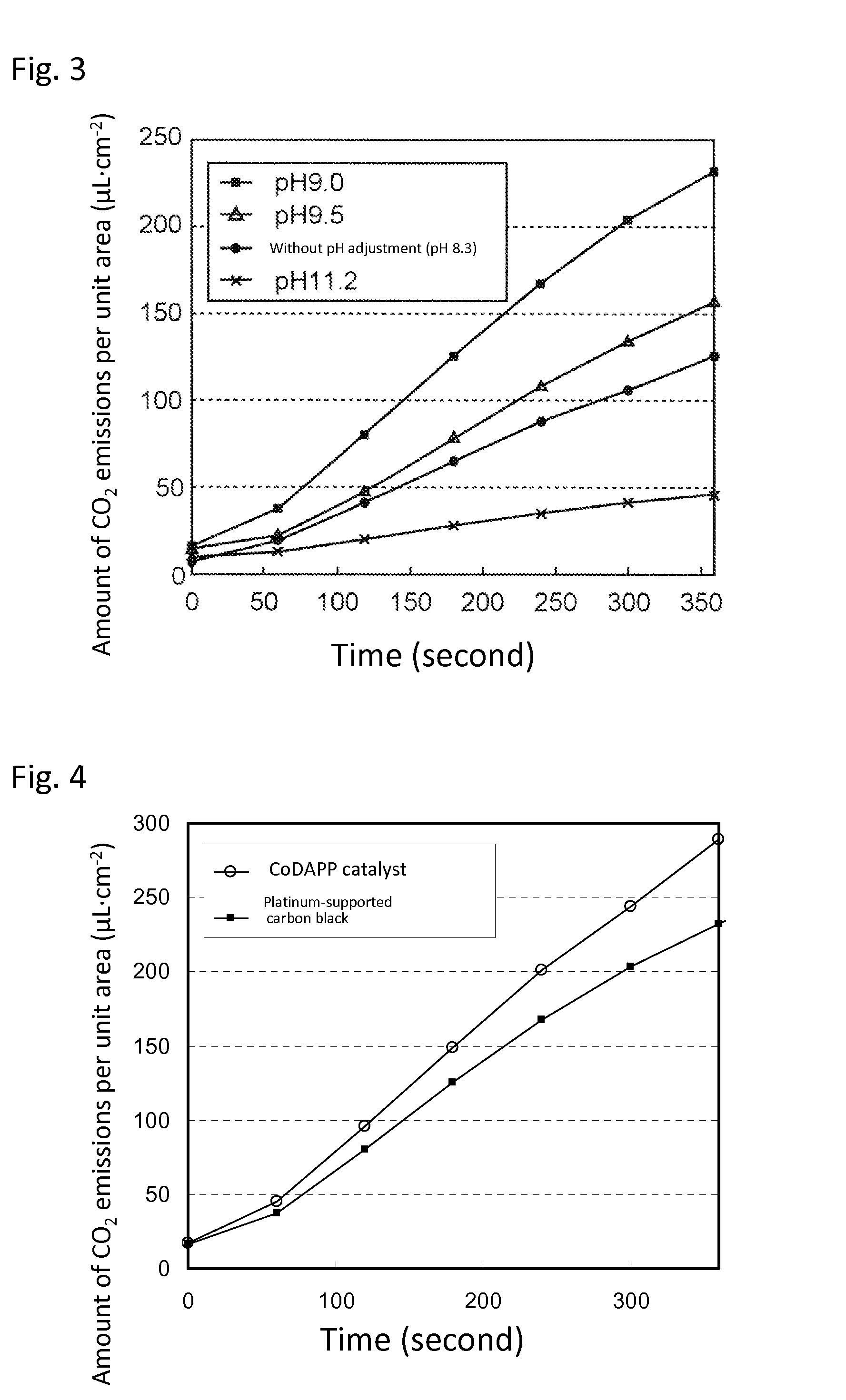
Carbon dioxide enrichment device
a carbon dioxide and enrichment device technology, applied in the field of carbon dioxide enrichment devices, can solve the problems of large energy consumption, inability to achieve the enrichment performance of carbon dioxide and low energy consumption, etc., and achieve the effects of high carbon dioxide enrichment performance, excellent effect, and reduced energy required during driving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Gas Diffusion Electrode
[0072]A commercially available carbon paper (porosity of 70%, thickness of 0.4 mm) was used as a conductive porous material. In order to improve gas diffusivity, a solution containing 30% by weight of polytetrafluoroethylene (PTFE) dispersed therein was applied on one surface of the carbon paper by a bar coater method, and then the resin was fixed to the carbon paper by firing in a nitrogen atmosphere electric furnace at a temperature of 340° C. for 20 minutes, and thus allowing to undergo water repellent finishing.
[0073]A catalyst paste to be applied on the carbon paper was prepared in the following manner. In a zirconia pot for ball mill, a commercially available platinum-supported carbon black (supporting 10 wt % Pt / Vulcan XC-72) was dispersed in 50 mL of a mixed solvent (2-propanol / water=1 / 1) so that the content of the carbon black becomes 100 mg. While stirring the dispersion, a commercially available PTFE was added dropwise and mixed in the...
example 2
[0078]In the same manner as in Example 1, the pH of the electrolytic solution 3 was variously changed by the addition of NaOH and DC voltage of 1.2 V was applied between the gas diffusion electrode 1 and gas diffusion electrode 2. The amount of carbon dioxide emissions was confirmed by measuring the concentration of carbon dioxide in the glass container attached to the gas diffusion electrode 2 using a carbon dioxide detector. The results are illustrated in FIG. 3. FIG. 3 revealed that the discharge rate of carbon dioxide significantly depends on the pH of the electrolytic solution pH, and that the amount of CO2 emissions per unit area becomes the maximum when the pH of the electrolytic solution is 9.0. As is apparent therefrom, the pH of the electrolytic solution 3 is preferably from 8.5 to 9.5.
example 3
Preparation of Electrode Catalyst (Co-2,6-diaminopyridine Polymer (CoDAP) Catalyst) Made of Metal Complex
[0080]A 2,6-diaminopyridine monomer (Aldrich Corporation) was mixed with an oxidizing agent ammonium peroxydisulfate (APS) (Wako Corporation) in a molar ratio of 1:1.5, followed by mixing. Specifically, 5.45 g of 2,6-diaminopyridine and 1 g of sodium hydroxide were dissolved in 400 mL of distilled water, and then 27.6 g of APS and 100 mL of water were added. The obtained mixture was stirred for 5 minutes and 2,6-diaminopyridine was polymerized at room temperature for 12 hours. After polymerization reaction, the obtained black precipitate was recovered by centrifugation at 3,000 rpm, and then washed three times with distilled water. The precipitate was dried under vacuum at 60° C. for several hours to obtain a 2,6-diaminopyridine polymer.
[0081]Subsequently, 5.45 g of a 2,6-diaminopyridine polymer and 3.62 g of cobalt nitrate (Wako Pure Chemical Industries, Ltd.) were suspended in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com