Compound having substituted anthracene ring structure and pyridoindole ring structure and organic electroluminescence device
a technology of pyridoindole and pyridoidole, which is applied in the direction of luminescent compositions, organic chemistry, thermoelectric devices, etc., can solve the problems of low electron transport properties, reduce efficiency, and cannot consider the hole-blocking capability of materials, etc., and achieve excellent hole-blocking ability, excellent thermal resistance, and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
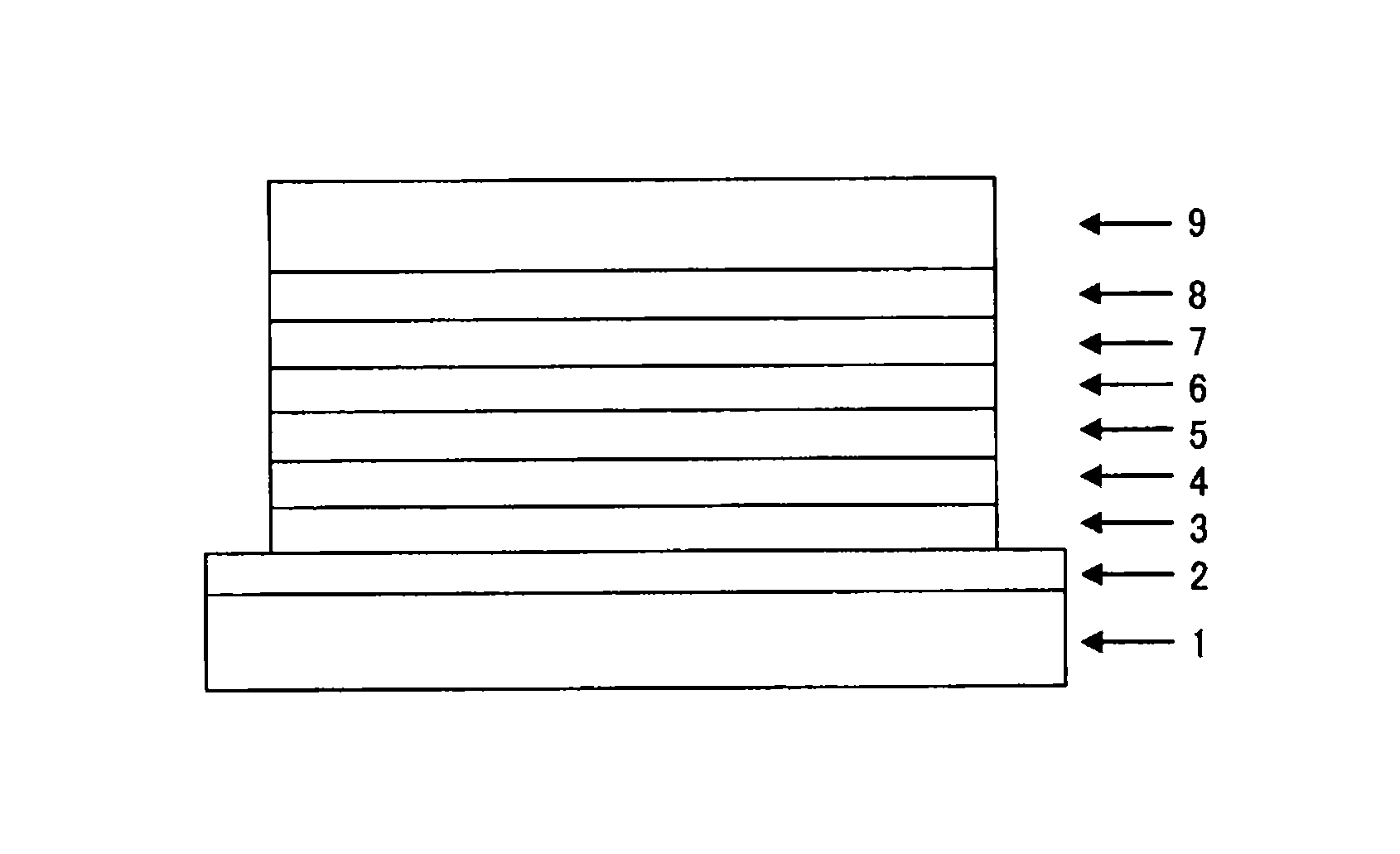
Image
Examples
example 1
Synthesis of 9,10-Diphenyl-2-(5H-pyrido[4,3-b]indol-5-yl)anthracene (Compound 3)
[0070]In a nitrogen atmosphere, 4.7 g of 2-bromo-9,10-diphenylanthracene, 2.6 g of 5H-pyrido[4,3-b]indole, 0.4 g of copper powder, 4.3 g of potassium carbonate, 0.3 ml of dimethyl sulfoxide and 30 ml of n-dodecane were added to a reaction vessel, then heated and stirred at 210° C. for 18 hours. After cooling to room temperature, 300 ml of chloroform and 30 ml of methanol were added thereto and insoluble materials were removed by filtration. The filtrate was concentrated under reduced pressure to obtain a crude product, and the crude product was purified by column chromatography (carrier: NH silica gel, eluent: toluene / chloroform) to obtain 1.7 g (yield: 30%) of 9,10-diphenyl-2-(5H-pyrido[4,3-b]indol-5-yl)anthracene (Compound 3) as a yellow powder.
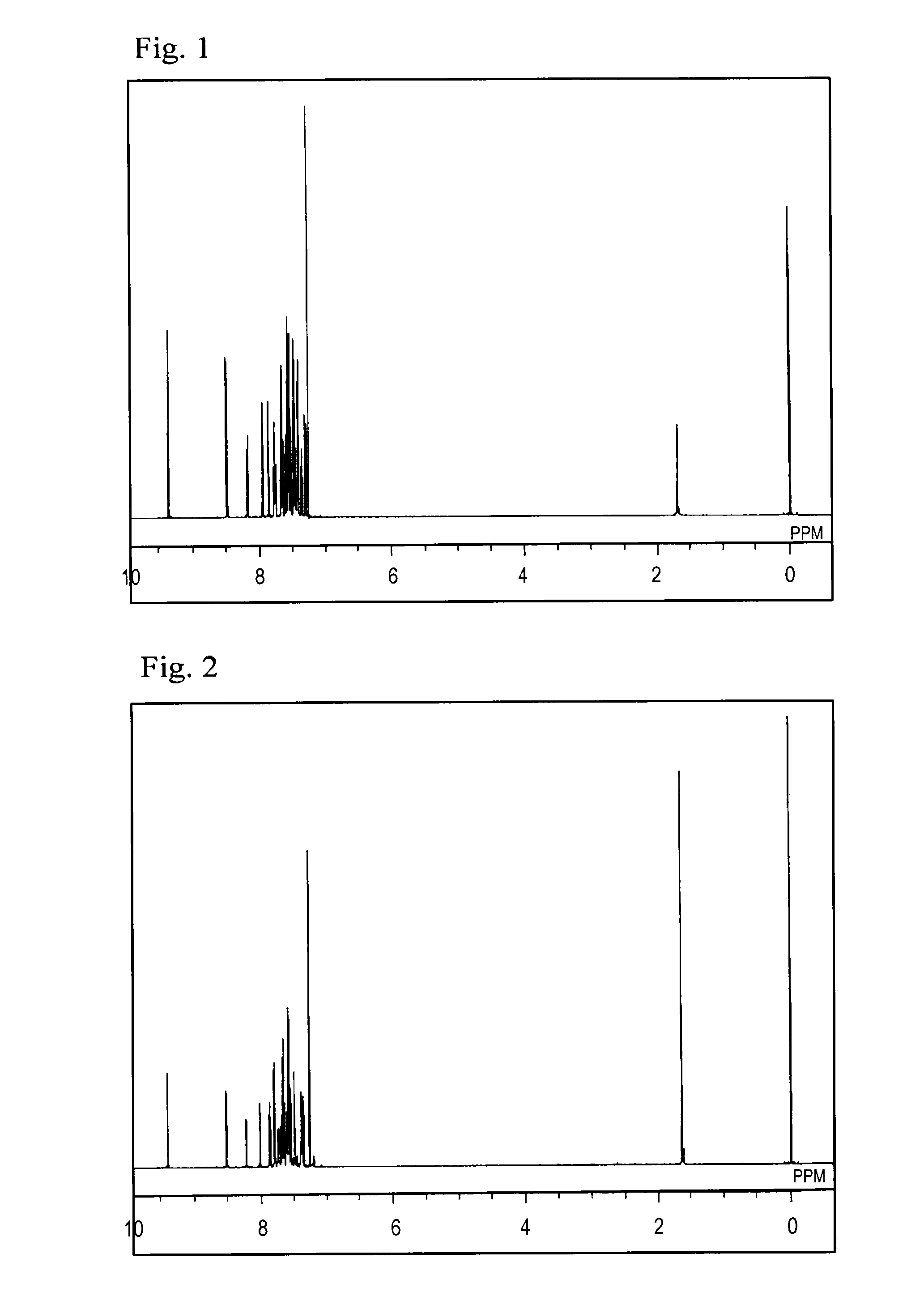
[0071]The structure of the obtained yellow powder was identified using NMR. FIG. 1 shows the results of 1H-NMR measurement.
[0072]The following 24 hydrogen signa...
example 2
Synthesis of 9,10-Diphenyl-2-[4-(5H-pyrido[4,3-b]indol-5-yl)phenyl]anthracene (Compound 9)
[0073]In a nitrogen atmosphere, 4.7 g of 9,10-diphenylanthracene-2-boronic acid, 2.8 g of 5-(4-bromophenyl)-5H-pyrido[4,3-b]indole, 0.50 g of tetrakistriphenylphosphine palladium, 15 ml of an aqueous 2 M potassium carbonate solution, 20 ml of toluene and 1.5 ml of ethanol were added to a reaction vessel, then heated under reflux with stirring for 18 hours. After cooling to room temperature, a crude product was collected by filtration, and the crude product was purified by column chromatography (carrier: silica gel, eluent: toluene / ethyl acetate) to obtain 1.6 g (yield: 32%) of 9,10-diphenyl-2-[4-(5H-pyrido[4,3-b]indol-5-yl)phenyl]anthracene (Compound 9) as a yellow powder.
[0074]The structure of the obtained yellow powder was identified using NMR. FIG. 2 shows the results of 1H-NMR measurement.
[0075]The following 28 hydrogen signals were detected on 1H-NMR (CDCl3). δ (ppm)=9.39 (1H), 8.52 (1H), ...
example 3
Synthesis of 9,10-Diphenyl-2-[6-(5H-pyrido[4,3-b]indol-5-yl)pyridin-3-yl]anthracene (Compound 15)
[0076]In a nitrogen atmosphere, 4.5 g of 9,10-diphenylanthracene-2-boronic acid, 3.0 g of 5-(5-bromopyridin-2-yl)-5H-pyrido[4,3-b]indole, 0.55 g of tetrakistriphenylphosphine palladium, 19 ml of an aqueous 2 M potassium carbonate solution, 72 ml of toluene and 18 ml of ethanol were added to a reaction vessel, then heated under reflux with stirring for 18 hours. After cooling to room temperature, 100 ml of toluene and 100 ml of water were added thereto, followed by stirring, and the organic layer was separated by liquid separation. The organic layer was dried over magnesium sulfate, and then concentrated under reduced pressure to obtain a crude product. The crude product was purified by column chromatography (carrier: NH silica gel, eluent: toluene) to obtain 3.0 g (yield: 57%) of 9,10-diphenyl-2-[6-(5H-pyrido[4,3-b]indol-5-yl)pyridin-3-yl]anthracene (Compound 15) as a yellow powder.
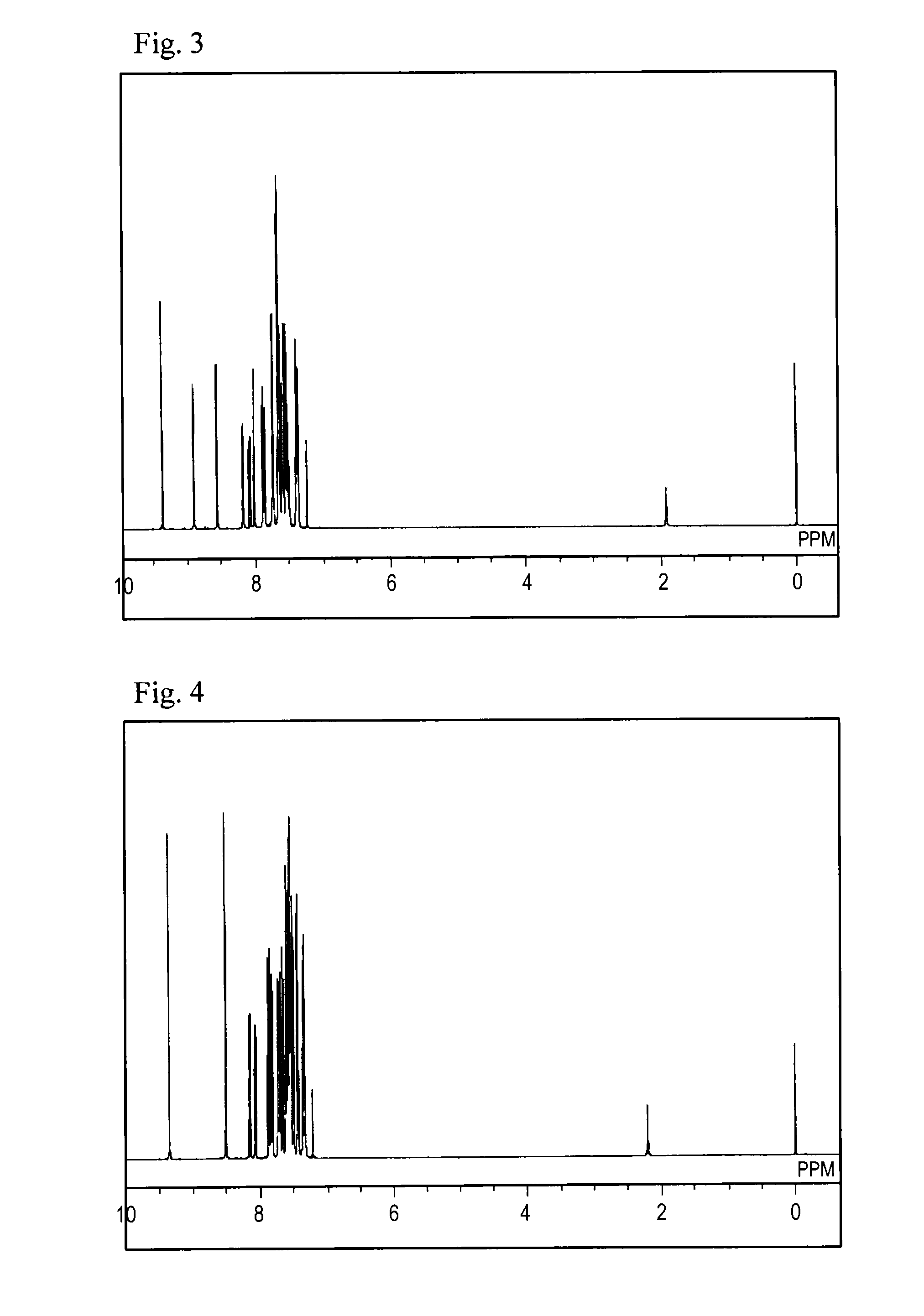
[0077...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com