Methods for the regulation of the prostaglandin f synthase (PGFS) activity of akr1b1 and uses thereof
a technology of prostaglandin f synthase and akr1b1, which is applied in the field of methods for the regulation of the prostaglandin f synthase (pgfs) activity of akr1b1, can solve the problems of inability to fully realize the effect of pgfs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Human AKR1B1 Qualifies as a Functional PGFS in the Endometrium
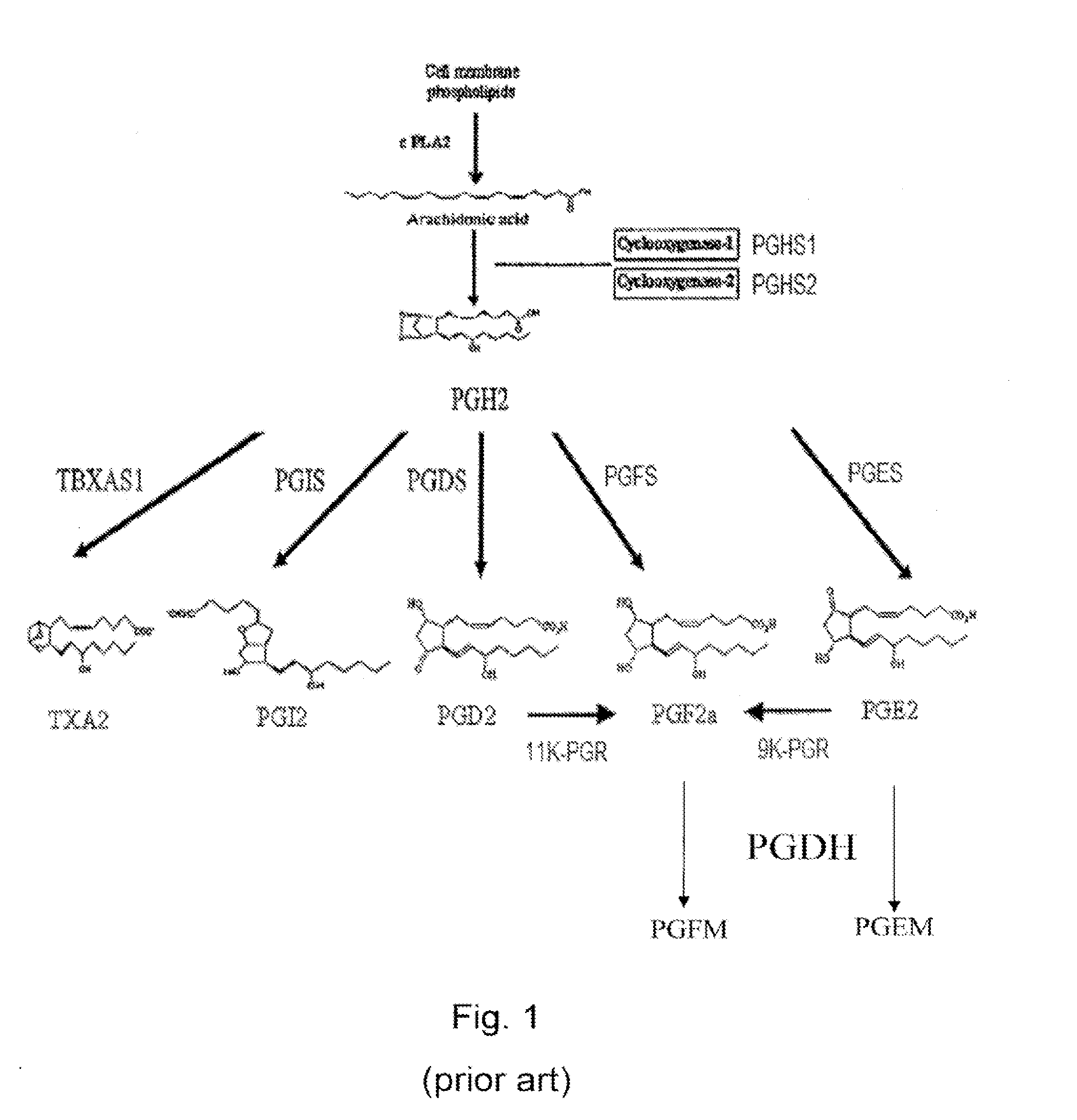
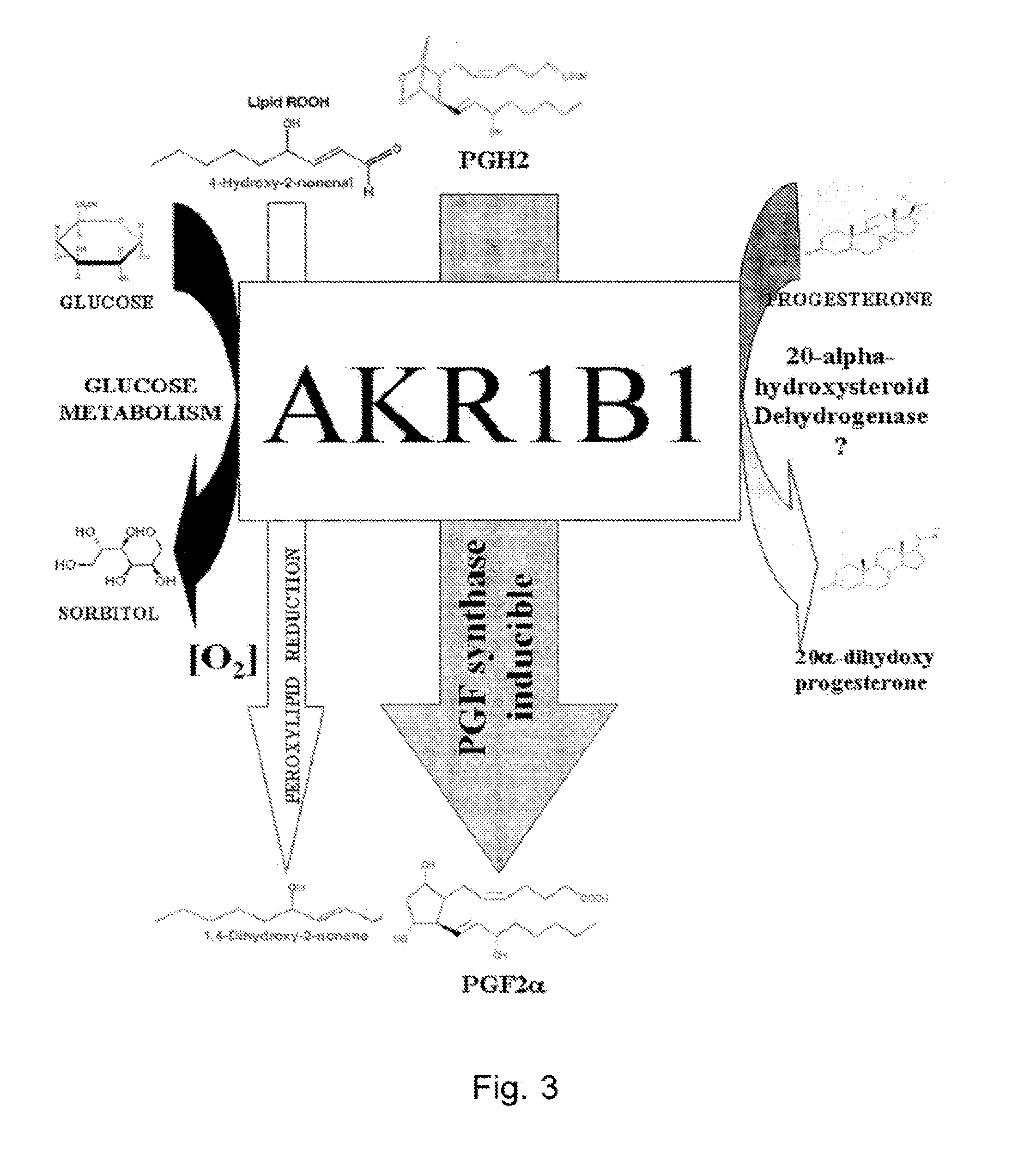
[0116]In the bovine endometrium, we previously demonstrated a strong PGFS activity of AKR1B5 recently renamed as bos taurus AKR1B1 (Gene ID: 317748), a new function for this enzyme previously known for its 20α-HSD and glucose metabolism activities (Madore et al., J Biol Chem 278(13); 11205-12, 2003). The human and bovine AKR1B1 both belong to the aldoketoreductase 1B family and share 86% identity or homology. The human AKR1B1 (Gene ID: 231) also known as the aldose reductase is highly expressed in the placenta for glucose metabolism and in the eye and kidney for osmotic regulation.
[0117]After identifying the bovine AKR1B1 as a functional PGFS, we have found that AKR1B1 expression was associated with PGF2α production in human endometrial cell lines and in decidualized stromal cells (Chapdelaine et al., Mol Hum Reprod, 12(5); 309-19, 2006). However, expression of AKR1B1 within the human endometrium and its ability to ac...
example 2
Retrovirus Infection and Establishment of SV40 Tag Cell Lines Expressing PGFS Activity
[0140]The retroviral vector SSR69 containing SV40 large TAG and a gene resistant to hygromycin was transfected with Effectene™ (Qiagen, Mississauga, ON, Canada) in the mouse amphotropic packaging cell line PA 317. The resulting colonies resistant to hygromycin (800 μg / ml, Roche, Mississauga, ON, Canada) were cultured, and the supernatants containing amphotropic viruses were collected and used to infect, separately, purified stromal and epithelial cells in primary culture. Endometrial cells grown in six-well plates were infected in the presence of polybrene (8μg / ml, Sigma) for 6 h, and the procedure was repeated 24 h later. The day following the last infection, the cells were trypsinized and seeded in 10 mm dishes in the presence of hygromycin (400 μg / ml). The cultures were grown for 7-8 days until the TAG-infected cells formed colonies while control non-infected cells died in the presence of the an...
example 3
Establishment of a Link Between AKR1B1 and Cox-2-Inhibitor-Associated Increased Risk of Heart Failure
[0143]Following the discovery of AKR1B1 as a major PGFS involved in the synthesis of PGF2α, and since AKR1B1 has been involved in diabetes-associated pathologies, and that its impact on cardiac and cerebral ischemia has been demonstrated in transgenic mice (Hwang Y. C. et al., 2004; Iwata K. et al., 2006; Vikramadithyan RK), we investigated if the PGFS function of AKR1B1 could allow for the identification of PGF2α as a molecule responsible for ischemia and pain.
[0144]The PGFS activity of AKR1B1 therefore represents a crucial step in the synthesis of PGF2α from PGH2 released by COX-1 and COX-2. The COX-inhibiting NSAIDs are commonly used in the treatment of headaches and muscle aches. Prior art studies of AKR1B1 mainly focused on its role in polyols synthesis or in lipid detoxification. While AKR1B1 inhibitors have been developed to treat pathological conditions such as diabetic compl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com