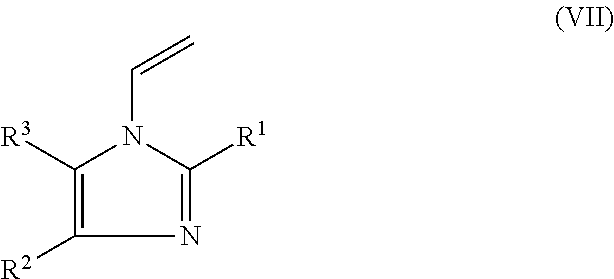
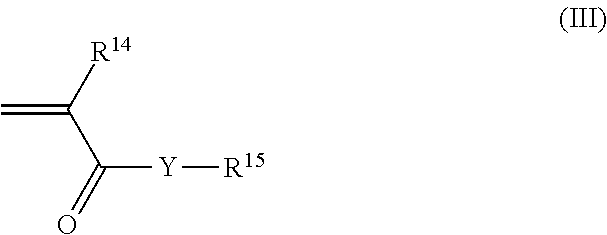Hair setting compositions based on t-butyl acrylate and hydroxyalkyl methacrylate
a technology of hydroxyalkyl methacrylate and tbutyl acrylate, which is applied in the direction of hair cosmetics, make-up, pharmaceutical delivery mechanisms, etc., can solve the problems of not having the required mechanical quality, affecting the effect of hair color, and affecting the appearance of the hair
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
tert-butyl acrylate / methacrylic acid 75 / 25 w / w (comparative example)
[0440]In a 2 l polymerization vessel with stirrer and heating and cooling devices, at a temperature from 20 to 25° C.
250 g of deionized water0.6 gof a 15% strength by weight aqueous solution ofsodium lauryl sulfate in deionized water 35 gof feed II (see below)
were initially introduced and heated to 45° C. with stirring and under a nitrogen atmosphere. After the temperature had been reached, feed I (see below) was added over the course of 5 minutes.
[0441]The mixture was then heated to 80° C. and, while stirring and maintaining the reaction temperature, the remainder of feed II was metered in over the course of 2.5 hours with constant feed streams.
[0442]When the feeds were complete, the reaction mixture was stirred for a further hour at 80° C. and then cooled to 60° C.
[0443]While maintaining the temperature of 60°, feed III (see below) was added. The mixture was then cooled to 35° C. and, while maintaining the reactio...
example 8
Preparation of Polymer 8 (Solution Polymerization in Ethanol)
[0450]The following feeds were prepared at 20° C. with stirring:
Feed 1: 123 g t-BA55 gt-BMA41 gHEMA15 gMAA 5 gAA200 g EthanolFeed 2: 7 gWako V 5950 gEthanol
[0451]At 20° C., a mixture of 300 g of ethanol, 15% of the total amount of feed 1, and 15% of the total amount of feed 2 were prepared. The mixture was heated to 78° C. under atmospheric pressure. After 78° C. had been reached, feed 1 and feed 2 were started at the same time. Feed 1 was metered in over the course of 3 h, and feed 2 was metered in over the course of 4 h with a constant feed stream. The reaction mixture was maintained at 78° C. throughout the entire feed. When feed 2 was complete, the reaction mixture was kept at 78° C. for a further 2 h, then cooled to room temperature.
example 9
Preparation of Polymer 9 (Solution Polymerization in Ethanol / Water with Water-Soluble Starters)
[0452]The following feeds were prepared at 20° C. with stirring:
Feed 1: 90 gMMA 30 gHEMA 30 gMAA200 gEthanolFeed 2: 3 gSodium peroxodisulfate135 gEthanol102 gWater
[0453]At 20° C., a mixture of 60 g of ethanol, 45 g of water, 15% of the total amount of feed 1, and 15% of the total amount of feed 2 were prepared. The mixture was heated to 78° C. under atmospheric pressure. After 78° C. had been reached, feed 1 and feed 2 were started at the same time. Feed 1 was metered in over the course of 3 h and feed 2 was metered in over the course of 4 h with a constant feed stream. The reaction mixture was maintained at 78° C. throughout the entire feed. When feed 2 was complete, the reaction mixture was maintained at 78° C. for a further 2 h, then cooled to room temperature.
[0454]Details of the monomer composition in percent by weight,
Polymer fromexamplet-BAt-BMAHEMAHPMAMAAothers1 (comparative7525e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com