Therapeutic Inhibition of Granulocyte Function in Demyelinating Disease
a demyelinating disease and granulocyte technology, applied in the field of demyelinating disease therapy inhibition, can solve the problems of inability to determine a priori which patients to prescribe, the response of patient populations is not uniform, and clinicians may need to prescribe sequential expensive and time-consuming therapies
Inactive Publication Date: 2013-02-21
THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
View PDF1 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
The patent provides a method for treating inflammatory demyelinating diseases using inhibitors of granulocyte function, particularly elastase inhibitors. These diseases include multiple sclerosis, neuromyelitis optica, and animal models of these diseases. The patent also discusses the use of pharmaceutical formulations containing elastase inhibitors and the importance of assessing patients' responsiveness to treatment. The patent also provides a method for identifying responder and non-responder patients with these diseases using a cytokine measurement panel. The technical effect of the patent is improved care for patients with inflammatory demyelinating diseases by providing targeted therapies based on their responsiveness to treatment.
Problems solved by technology
A downside to this promising therapy is the diversity of responses in patient populations.
The clinician may therefore need to prescribe sequential expensive and time-consuming therapies in order to determine which is effective for the individual patient.
The use of disease-modifying therapies in autoimmune conditions is of great clinical interest; however these therapies suffer from the inability to determine a priori which patients will benefit.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
example 2
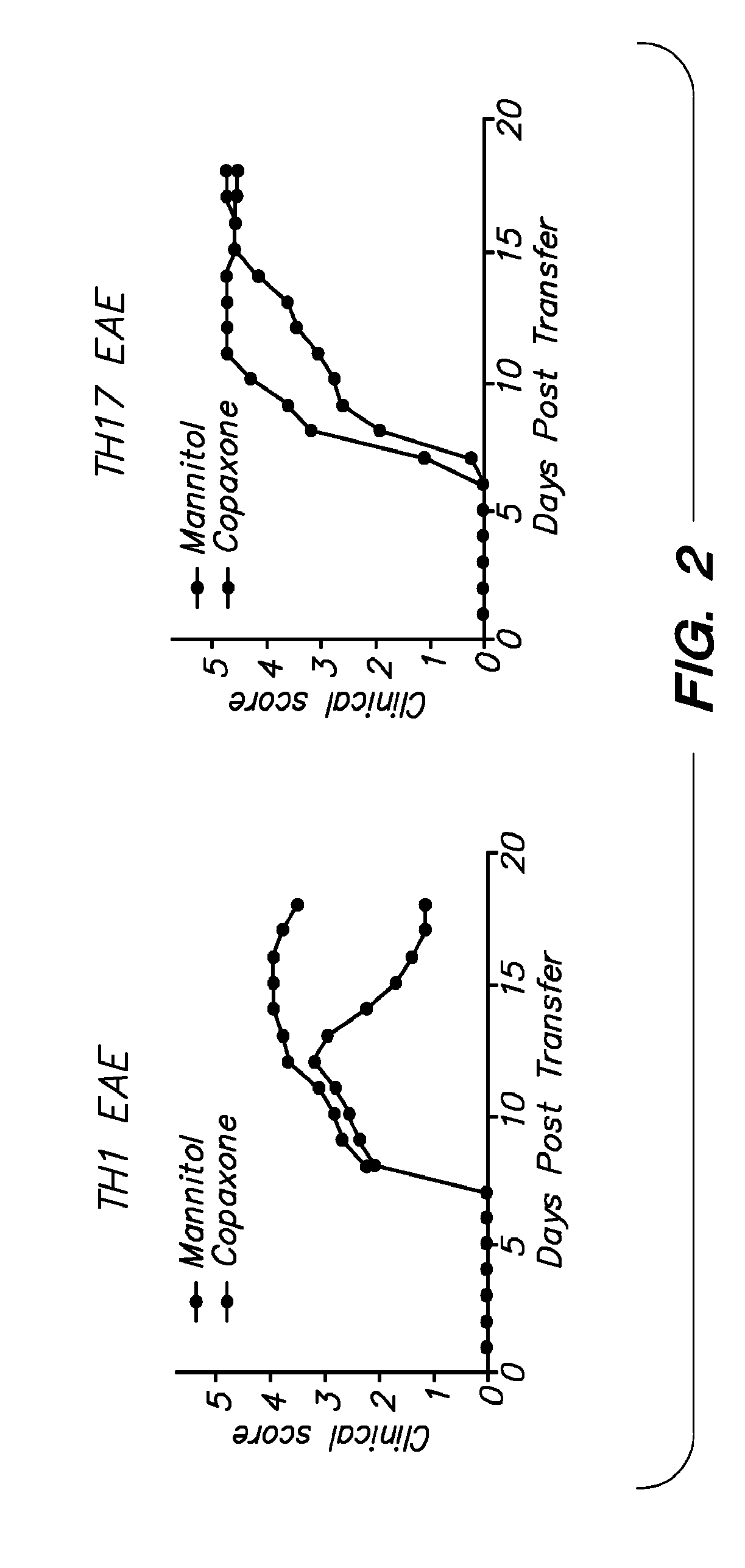
Increased Granulocytes are Indicative of NMO and TH17 EAE
[0094]As shown in FIG. 4A, markers indicative of granulocytes are found at increased levels in NMO and TH17EAE. In plasma, increased levels of neutrophil elastase is found in patients with NMO (FIG. 4B).
[0095]Remarkably, an elastase inhibitor attenuated TH17 EAE, shown in FIG. 4C, indicating the utility of this treatment for β-interferon resistant MS and NMO.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular mass | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Login to View More
Abstract
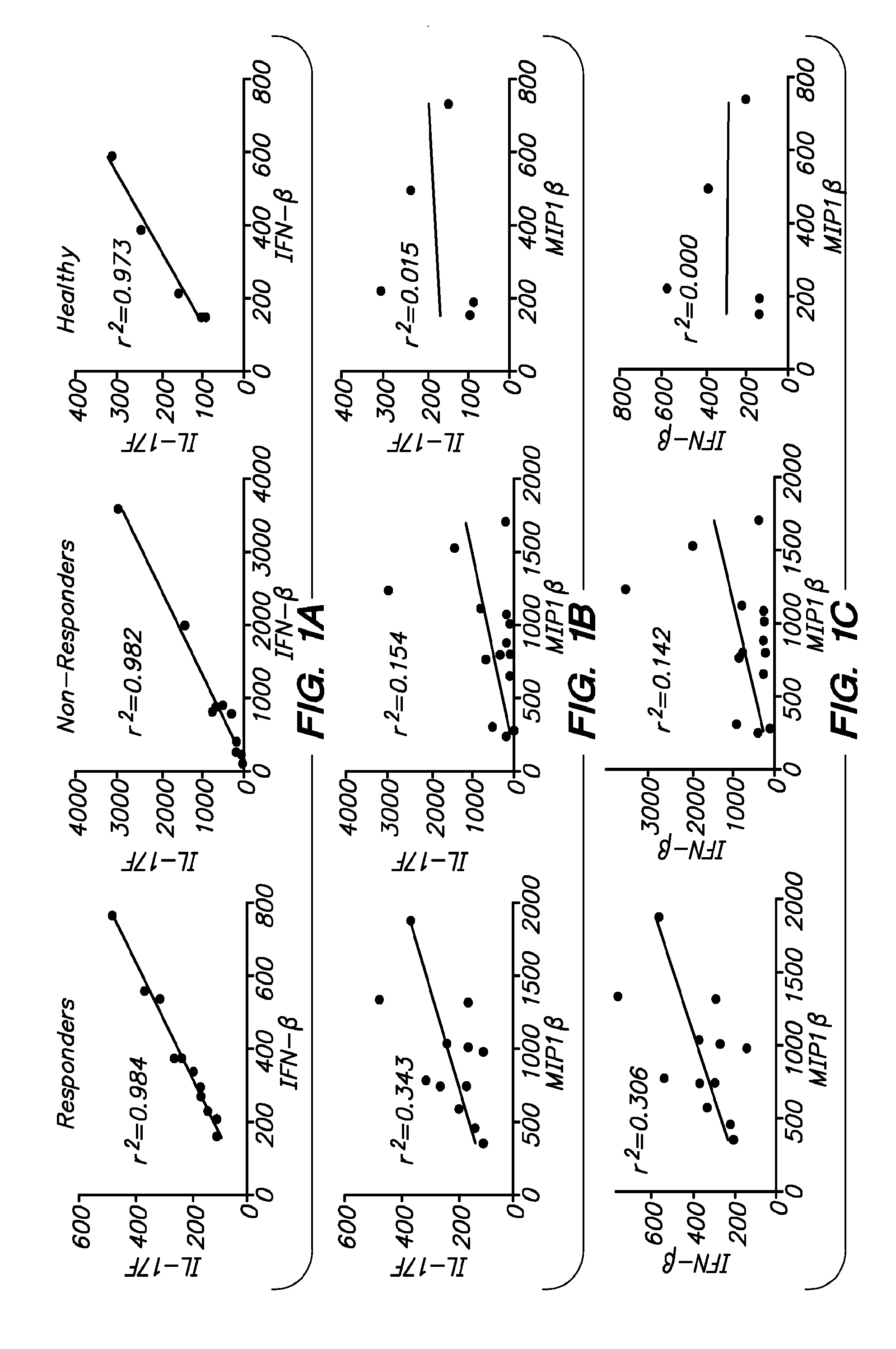
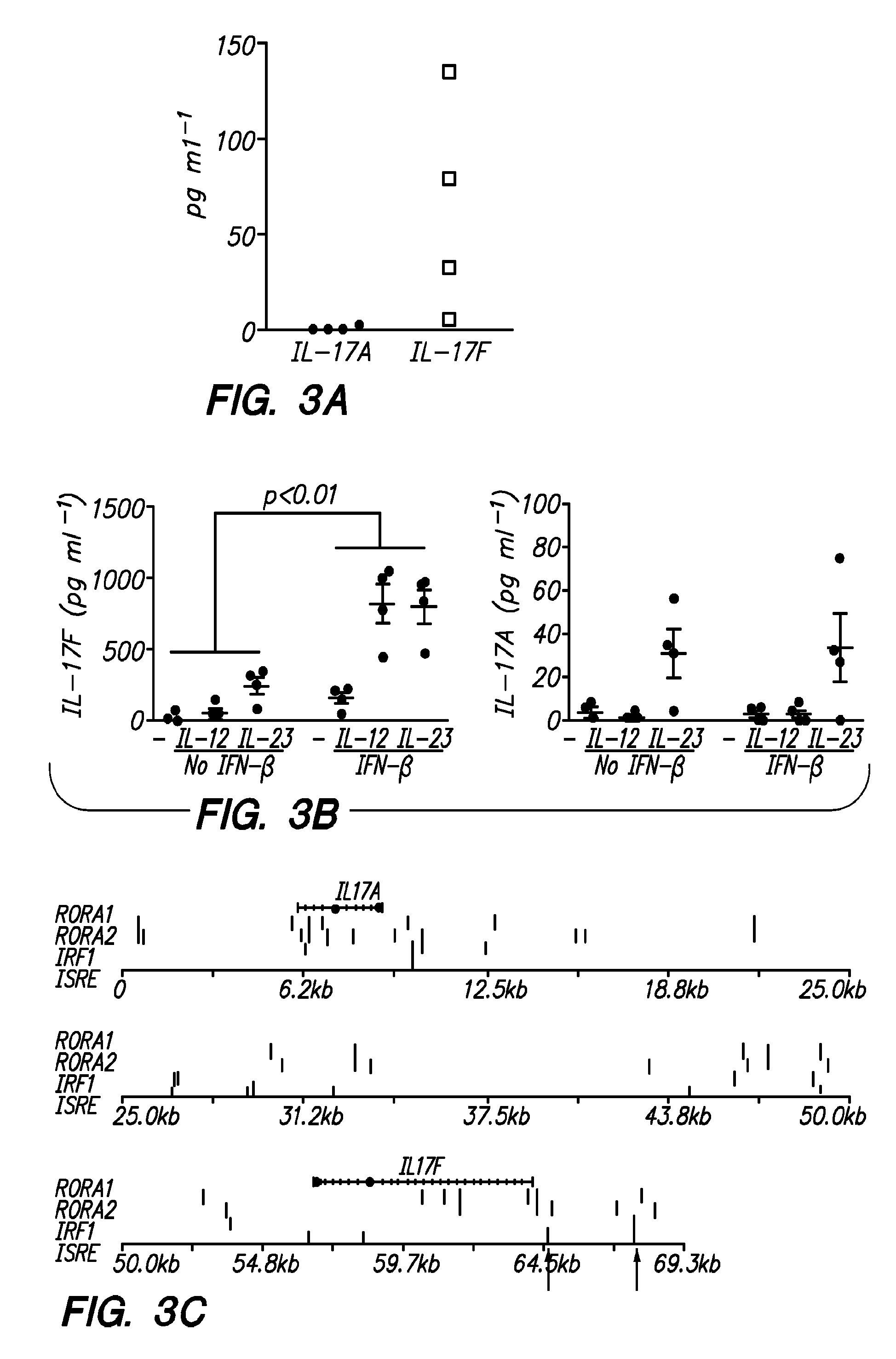
Compositions and methods are provided for the treatment of IL-17-type inflammatory demyelinating diseases with inhibitors of granulocyte function, e.g. elastase inhibitors. Diseases of interest include multiple sclerosis, neuromyelitis optica, animal models of such diseases, etc. In some embodiments pharmaceutical formulations comprising an elastase inhibitor in an effective dose for treatment of IL-17-type inflammatory demyelinating disease and a pharmaceutically acceptable excipient are provided. Patients may be classified into subtypes prior to treatment, which subtypes are informative of the patient's need for therapy and responsiveness to a therapy of interest.
Description
BACKGROUND OF THE INVENTION[0001]There is a long-standing interest in manipulating cells of the immune system to achieve control of autoimmune disease. While targeted antigen-specific therapy remains of great interest, there has also been considerable development of polyclonal, or non-antigen specific therapies. In addition to general immunosuppression, e.g. through the use of agents such as hydrocortisone, many therapies are now being brought to the clinic that provide for a more selective modification of the immune system.[0002]A downside to this promising therapy is the diversity of responses in patient populations. While a significant proportion of patients may respond to a particular therapy, many do not. The clinician may therefore need to prescribe sequential expensive and time-consuming therapies in order to determine which is effective for the individual patient. Furthermore, it has been reported that IFN-β can exacerbate symptoms in some individuals.[0003]The use of diseas...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(United States)
IPC IPC(8): A61K31/225A61P25/00A61P29/00
CPCA61K31/18A61K31/225G01N2800/285G01N33/6896G01N2333/54G01N33/6863A61P1/16A61P11/00A61P11/06A61P25/00A61P25/16A61P29/00A61P3/04A61P9/10A61P3/10Y02A90/10
Inventor AXTELL, ROBERT C.STEINMAN, LAWRENCE
Owner THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com