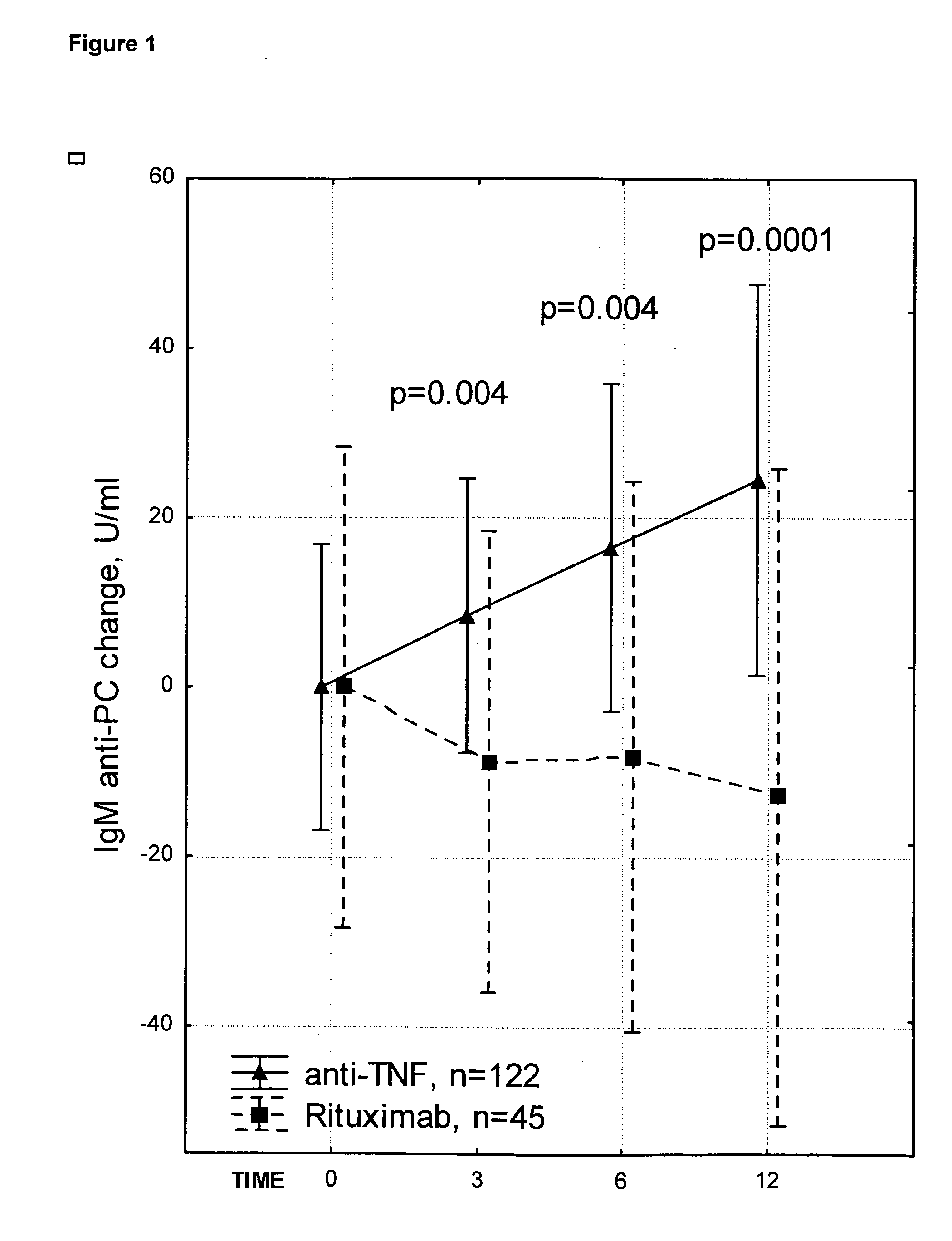
Antibodies Against Phosphorylcholine In Combination Therapy with Biologic Agents
a technology of phosphorylcholine and biologic agents, which is applied in the direction of drug compositions, immunological disorders, metabolism disorders, etc., can solve the problems of increased risk of cvdsup>16-18 etc., to achieve anti-inflammatory properties, low levels of anti-pc, and lack of response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Inhibition of Lysophosphatidylcholine Induced Cell Death by Anti-PC IgM
[0117]Peripheral blood mononuclear cells (PBMC) in 96 well plates (with 300,000 cells / well) were incubated with lysophosphatidylcholine (LPC) for 2 h, and cell death was assessed by measuring lactate dehydrogenase (LDH) activity in the supernatant. Antibodies against phosphorylcholine (anti-PC IgM) or control antibodies which did not bind PC, were preincubated with LPC 90 minutes before starting the treatment. The results were expressed as units per liter of LDH. As can be seen from FIG. 3, anti-PC IgM reduced cell death more than total IgM and flowthrough IgM.
[0118]PBMC (3,000,000 cells / well) were incubated with LPC for 18 h, and cell death was assessed by measuring the decrease in mitochondrial dependent reduction of MTT to formazin. Anti-PC, total IgM and IgM which did not bind PC (flow through) were preincubated with LPC 90 minutes before starting the treatment. The results were expressed as percentage of via...
example 3
Protective Effect of Combination of High Anti-PC with High Anti-MDA-LDL or High Anti-OxLDL
ELSA-study
Materials and Methods
Subjects
[0119]Serum samples were obtained from 226 subjects with established hypertension (diastolic pressure >95 mm Hg) prior to their entry into the Swedish component of the European Lacidipine Study on Atherosclerosis (ELSA)44, 45. Samples were collected following a 4-week washout period without medication to minimize the effects of treatment on the measured parameters. Blood pressure, cholesterol and triglyceride levels were determined as described previously44, 45 One hundred and fifteen of the subjects were subsequently assigned to treatment with the β-blocker atenolol, and 111 of the subjects were assigned to treatment with the calcium antagonist lacidipine.
[0120]Carotid ultrasound determinations were performed and analysed as detailed elsewhere19,44,45. A total of 226 patients had valid ultrasound measurements at baseline and after 4 years of follow up. Br...
example 4
Increased Risk of CVD from Combination of Low IgM Anti-PC with Low Anti-OxCL or Low Anti-OxPS
Study of 60-Year Olds
[0127]Objective.
[0128]We here determine the role of IgM antibodies against phosphorylcholine (anti-PC) in combination with antibodies against oxidized cardiolipin (anti-OxCL) or antibodies against oxidized phosphatidylserine (anti-OxPS) in prediction of cardiovascular disease (CVD).
[0129]Methods.
[0130]From a screening of 4232 subjects, 60-years old (2039 men and 2193 women), 211 incident cases of CVD (myocardial infarction, ischemic stroke, or hospitalized angina pectoris) and 633 age- and sex-matched controls were identified through a 5-7 year follow-up18,46,47. Serum levels of antibodies was determined by ELISA. Cardiolipin and phosphatidylserine was oxidized in aqueous solutions containing 1.5 mmol / L tert-butylhydroperoxide and CuSO4 in 20 μmol / L.
[0131]Results.
[0132]In women, there were no significant associations between anti-PC and CVD. Among men, individuals with a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diastolic pressure | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com