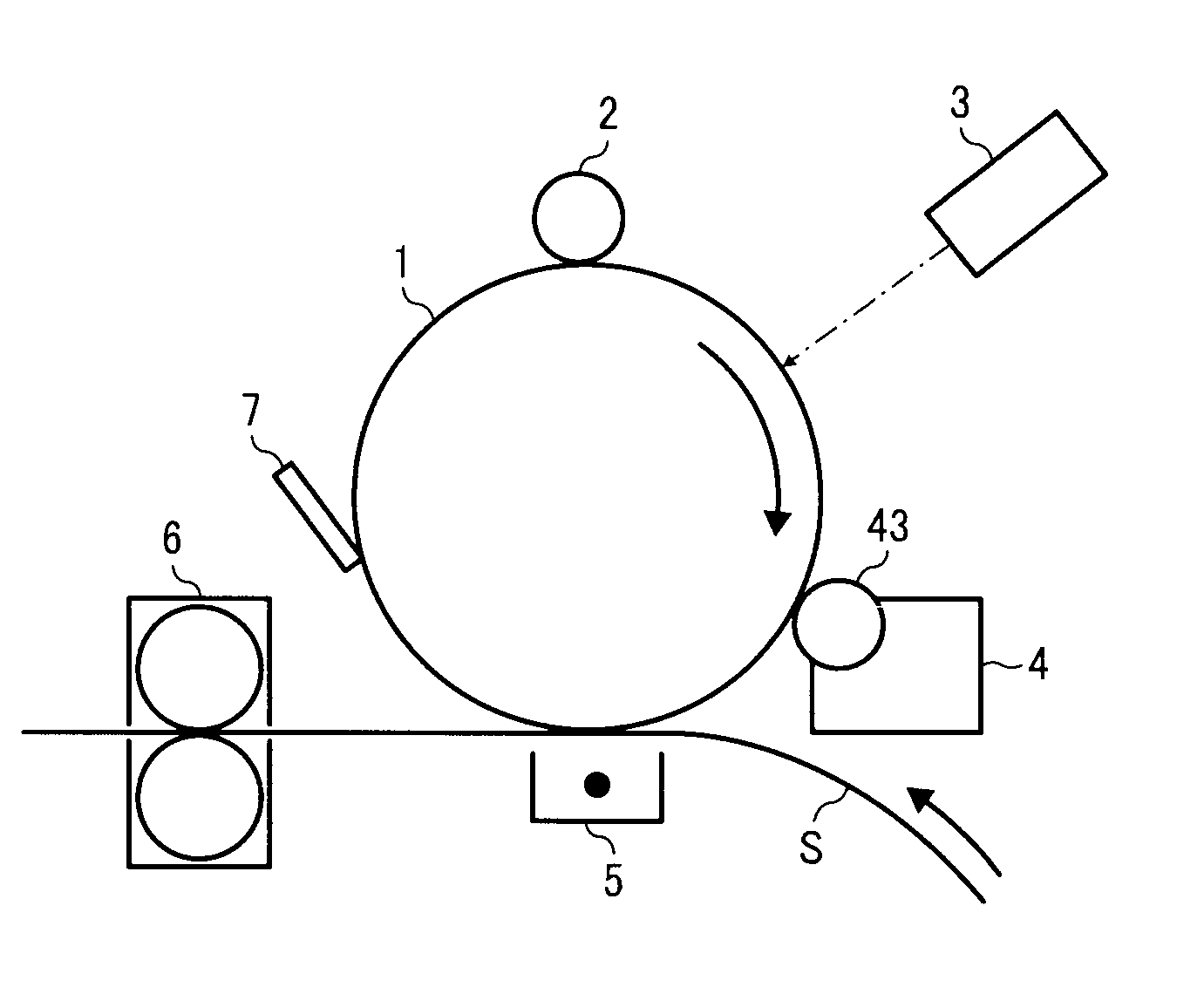
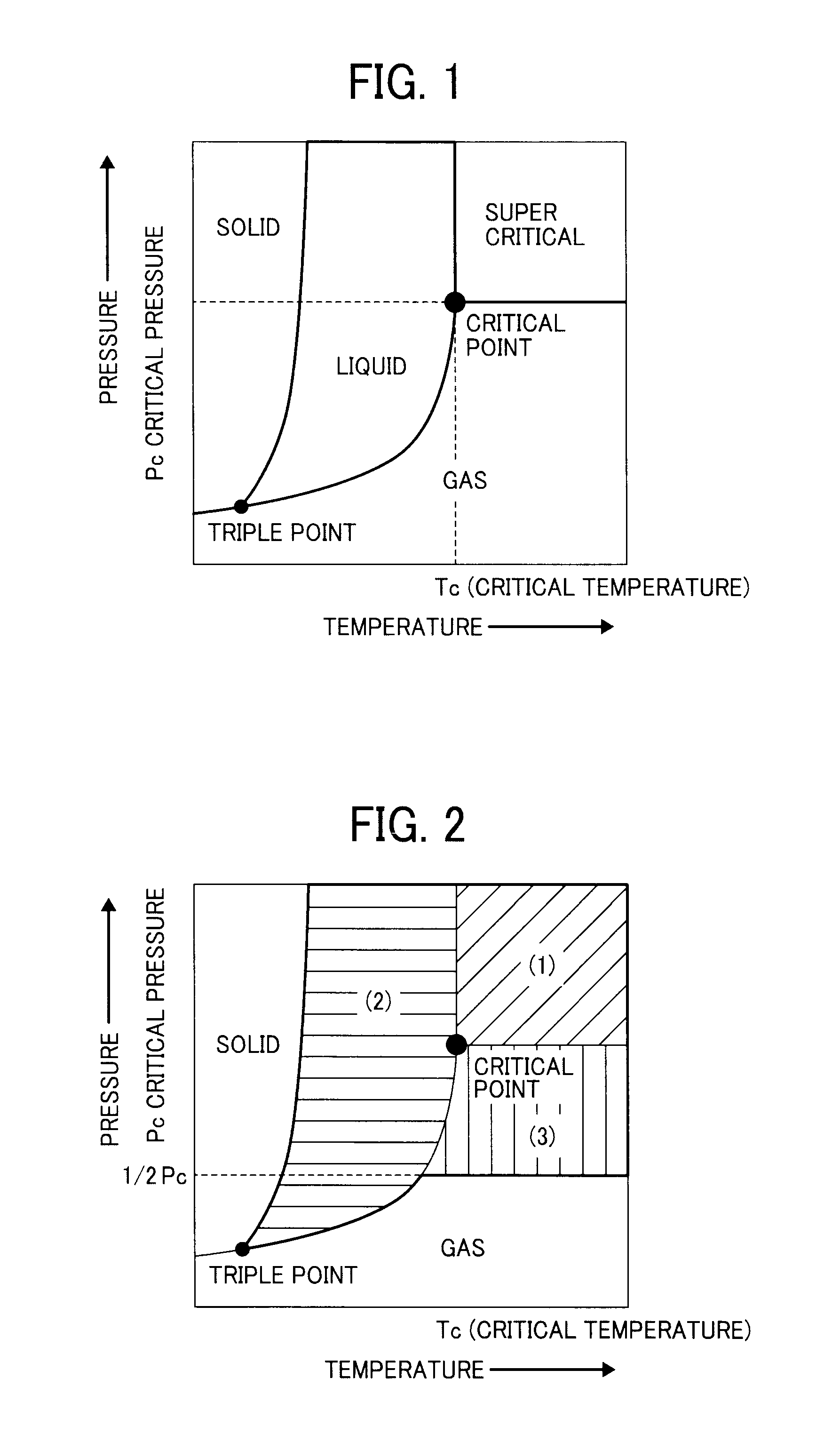
Toner
a technology of toner and abrasive, which is applied in the field of toner, can solve the problems of inability to obtain particulate polymers with small molecular weight distributions (mw/mn=about 2 or less), high cost of fluorochemical surfactants used in this method, and achieve the effect of small molecular weight distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
Synthesis of Surfactant 2
[0156]1,250 parts of 1H, 1H-perfluorooctylacrylate from AZ max. co. and 62.5 parts of 2,2′-azobis(2,4-dimethylvaleronitrile) V-6 from Wako Pure Chemical Industries, Ltd. were filled in a pressure resistant reaction cell (50 volume % of a pressure resistant container cell). Carbone dioxide as a supercritical fluid was fed into the reaction cell by a feed cylinder, and a reaction was performed for 24 hrs at 15 Mpa and 85° C. while controlled by a pressure pump and a heat regulator. Next, the temperature was decreased to 0° C. and the pressure was reduce to normal pressure using a back pressure valve to prepare a surfactant 2 having the following formula. The surfactant 2 had a number-average molecular weight (Mn) of 2,500.
wherein q is an integer representing a repeat unit.
synthesis example 2
Synthesis of Surfactant 3
[0157]In a 6-ml vial container, 36.1 parts of polyacrylate 5,000 from Wako Pure Chemical Industries, Ltd., 1,480 parts of chloroform from Wako Pure Chemical Industries, Ltd. and 128 parts of 1,1′-carbonylbis-1H-imidazole were placed and stirred for 10 min at room temperature.
[0158]Next, 500 parts of polyethylene glycol having a molecular weight of 200 from Wako Pure Chemical Industries, Ltd. were added thereto and stirred for 12 hrs.
[0159]Then, chloroform was added thereto, and washed with water.
[0160]Next, the mixture was dried with anhydrous sodium sulfate, filtered, and further vacuum-concentrated to prepare a surfactant 3 having the following formula (yield rate 73% by weight). The surfactant 3 had a number-average molecular weight (Mn) of 5,200.
wherein each of r and p is an integer representing a repeat unit.
synthesis example 3
Synthesis of Surfactant 4
[0161]In a 50-ml flask having the shape of an egg plant, 12 parts of side-chain carboxy-modified silicone oil KF-8012 having a molecular weight 4,500 from Shin-Etsu Chemical Co., Ltd., 33.3 parts of chloroform from Wako Pure Chemical Industries, Ltd., 1,1′-carbonylbis-1H-imidazole and 1.3 parts of polyethylene glycol having a molecular weight of 200 from Wako Pure Chemical Industries, Ltd. were placed and stirred for 12 hrs at room temperature.
[0162]Next, saturated sodium hydrogen carbonate aqueous solution was added to the mixture to precipitate sodium stearate, which was filtered with a Kiriyama funnel and washed with saturated sodium hydrogen carbonate aqueous solution.
[0163]Then, the mixture was dried with anhydrous sodium sulfate, filtered with a silica gel, and further vacuum-concentrated to prepare a surfactant 4 having the following formula (yield rate 91% by weight). The surfactant 3 had a number-average molecular weight (Mn) of 4,700.
wherein each o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distributions | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com