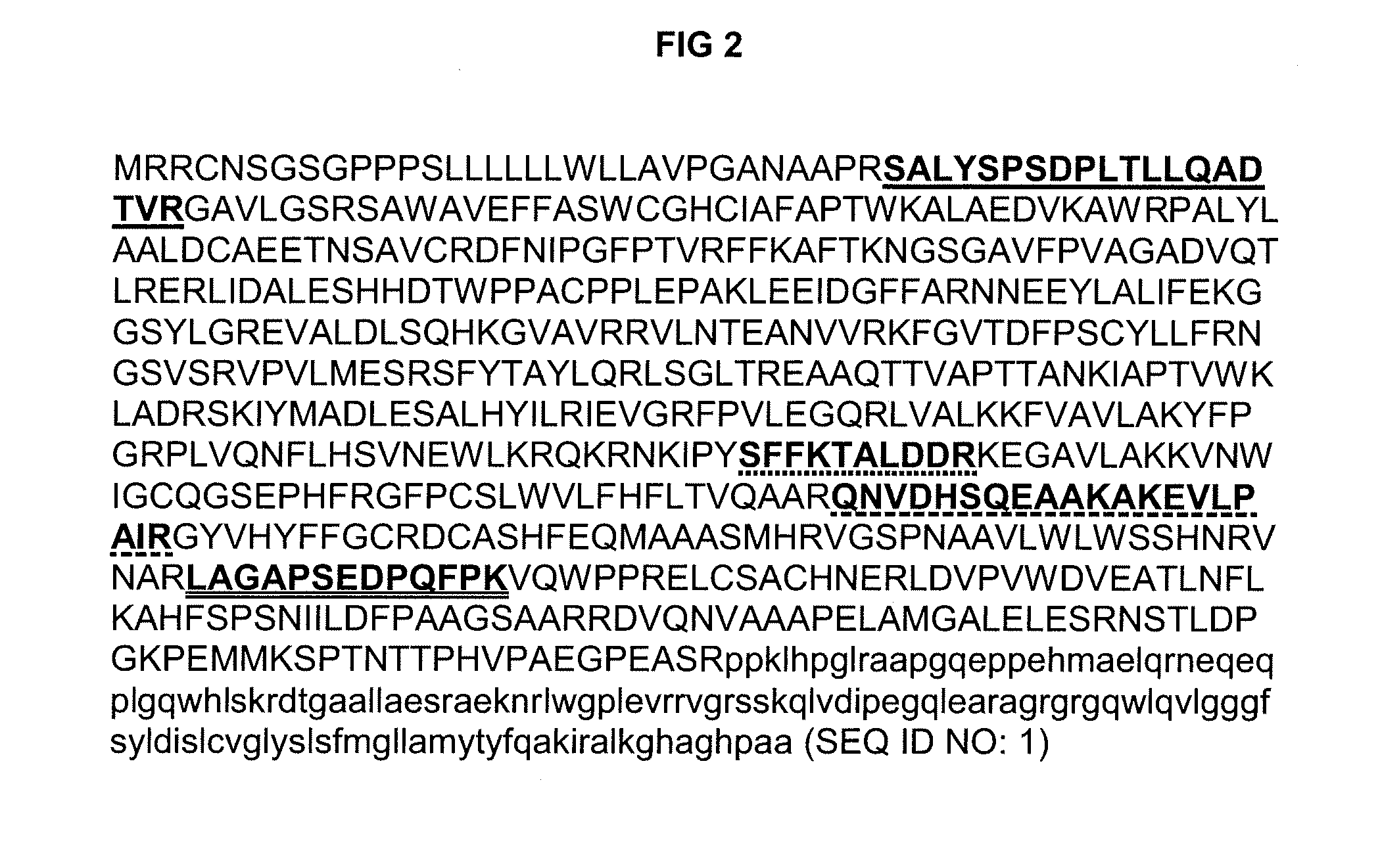
Biomarker for diagnosis, prediction and/or prognosis of acute heart failure and uses thereof
a biomarker and acute heart failure technology, applied in the field of proteinand/or peptide-based biomarkers, can solve the problems of unambiguous diagnosis, inability of acute heart failure to pump efficiently and where it cannot no longer, and considerable morbidity and mortality among older adults
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
MASSTERMIND Discovery Platform for Discovery of New Biomarkers for AHF
[0333]MASSTERMIND Experimental Setup
[0334]For biomarker discovery, we analysed the changes in protein expression using mass spectrometric detection of protein levels using our previously published COFRADIC™ technology platform (substantially as described inter alia in WO 02 / 077016 and in Gevaert et al. 2003, Nat Biotechnol 21(5): 566-9).
[0335]All plasma samples were depleted for the most abundant proteins using commercially available affinity-based chromatographic columns (e.g. Agilent Technologies). Depletion efficiency of albumin and immunoglobulin G (IgG) was checked using Western Blot analysis. Samples were prepared for MASStermind analysis according to the standard N-terminal COFRADIC procedures. Samples and controls were differentially labelled by trypsin mediated incorporation of 18O / 16O at the C-terminus of every tryptic peptide. After N-terminal peptide sorting, NanoLC separations followed by direct spott...
example 2
MASSterclass Targeted Protein Quantitation for Early Validation of Candidate Markers Derived from Discovery
[0338]MASSTERCLASS Experimental Setup
[0339]MASSterclass assays use targeted tandem mass spectrometry with stable isotope dilution as an end-stage peptide quantitation system (also called Multiple Reaction Monitoring (MRM) and Single Reaction Monitoring (SRM)). The targeted peptide is specific (i.e., proteotypic) for the specific protein of interest. i.e., the amount of peptide measured is directly related to the amount of protein in the original sample. To reach the specificity and sensitivity needed for biomarker quantitation in complex samples, peptide fractionations precede the end-stage quantitation step.
[0340]A suitable MASSTERCLASS assay may include the following steps:[0341]Plasma / serum sample[0342]Depletion of human albumin and IgG (complexity reduction on protein level) using affinity capture with anti-albumin and anti-IgG antibodies using ProteoPrep spin columns (Sigm...
example 3
Unbiased Discovery of Novel AHF Markers Using MASStermind
[0370]The MASStermind proteomic discovery platform was used to discover novel low abundance AHF protein biomarker candidates directly in patient plasma. Serial plasma samples collected prospectively from 10 patients with AHF on admission to the emergency department and just prior to their discharge from hospital were analyzed alongside age and gender matched control samples collected from healthy individuals (FIG. 4). Comparing protein profiles of AHF patients at admission versus at discharge yields biomarker candidates for treatment monitoring and discharge decisions while a comparison of AHF patients with healthy matched controls provides with new biomarker candidates for improving diagnostic accuracy.
[0371]Following the MASStermind procedure differential features that discriminate AHF and healthy populations and / or admission and discharge samples were selected by different statistical measures (SAM and one-rule classifier)....
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| mass spectrometry analysis | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com