Composite repair resins containing minimal hazardous air pollutants and volatile organic compound
a technology of volatile organic compounds and composite resins, which is applied in the direction of special tyres, vehicle components, tire parts, etc., can solve the problems of short shelf life of less than 1 year, large hap emissions, and thousands of pounds of hazardous waste generated by military resins annually
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
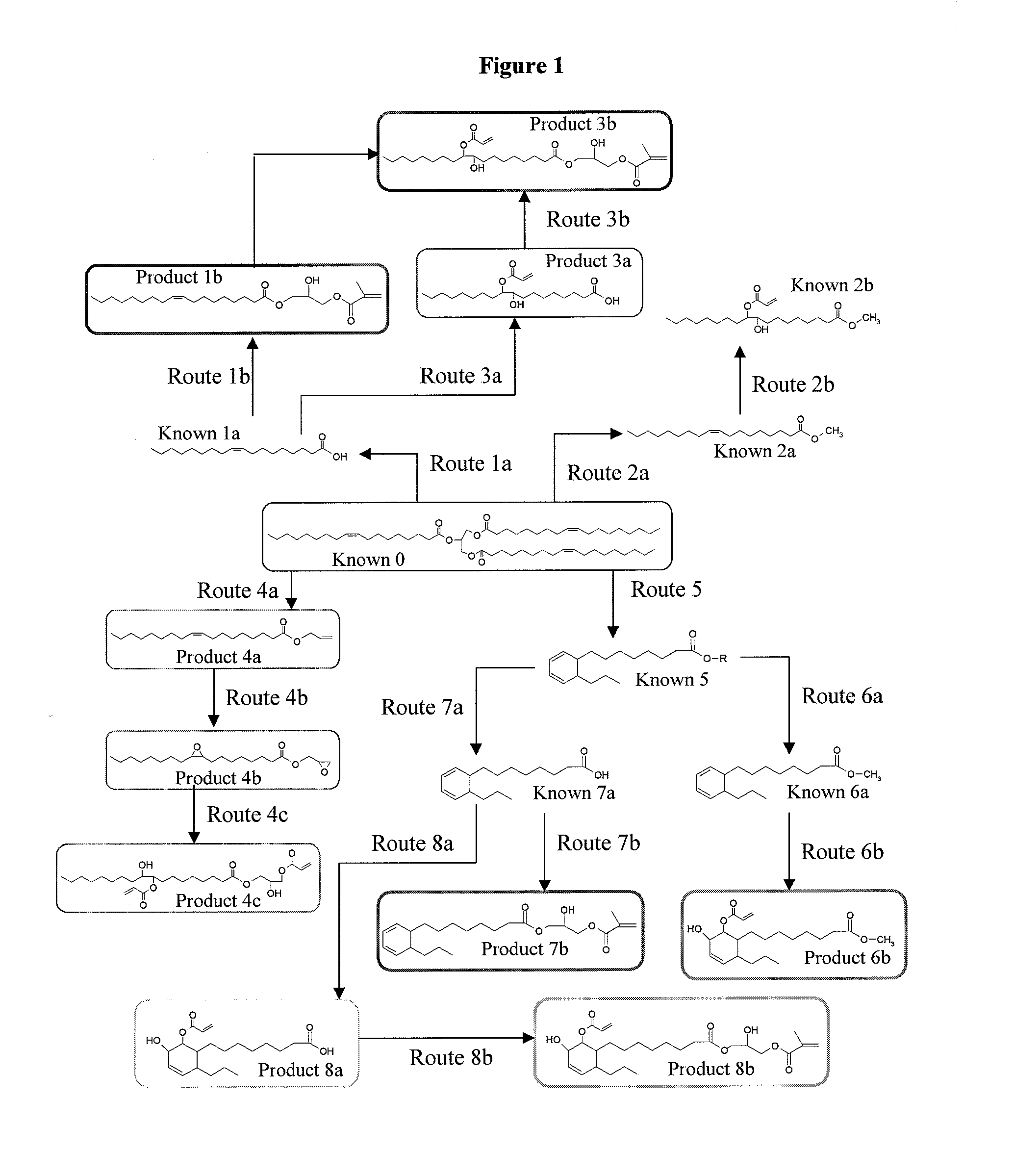
[0084]The reaction between a stoichiometric amount of oleic acid (OA) and glycidyl methacrylate (GM) was catalyzed with 2 wt % AMC-2™ catalyst and run at room temperature. The reaction went to ˜90% completion after 3 days of reaction.
examples 2-4
[0085]The reaction between a stoichiometric amount of oleic acid and glycidyl methacrylate was catalyzed with 0.5 wt %, 1 wt %, and 2 wt % AMC-2™ catalyst and run at 70° C. The reaction went to completion in 1.5 hrs when 2 wt % catalyst was used. The reaction went to completion after 2.5 hours when 1 wt % catalyst was used. When 0.5 wt % catalyst was used, the reaction took longer than 4 hours.
examples 5-7
[0086]The effect of higher reaction temperatures on the reaction of oleic acid and glycidyl methacrylate was studied using 1 wt % AMC-2™ catalyst. Reaction temperatures of 80° C., 90° C., and 100° C. were used. It was found that increasing temperature decreased the reaction time necessary to reach complete reaction of the epoxides. However, at 100° C., HPLC results show the formation of some higher molecular weight species. This indicates that undesirable epoxy homopolymerization (etherification) occurred to some extent at 100° C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com