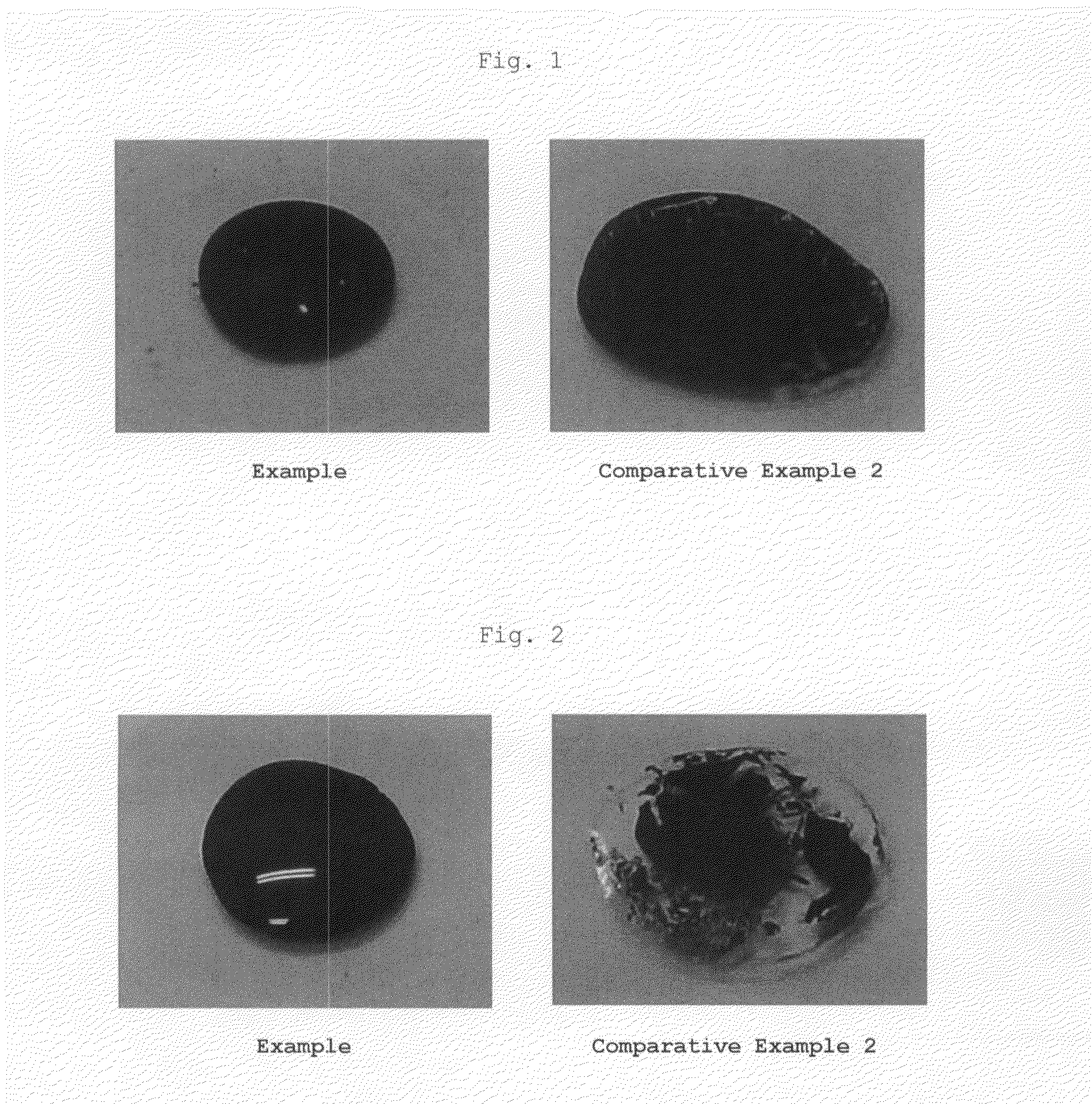
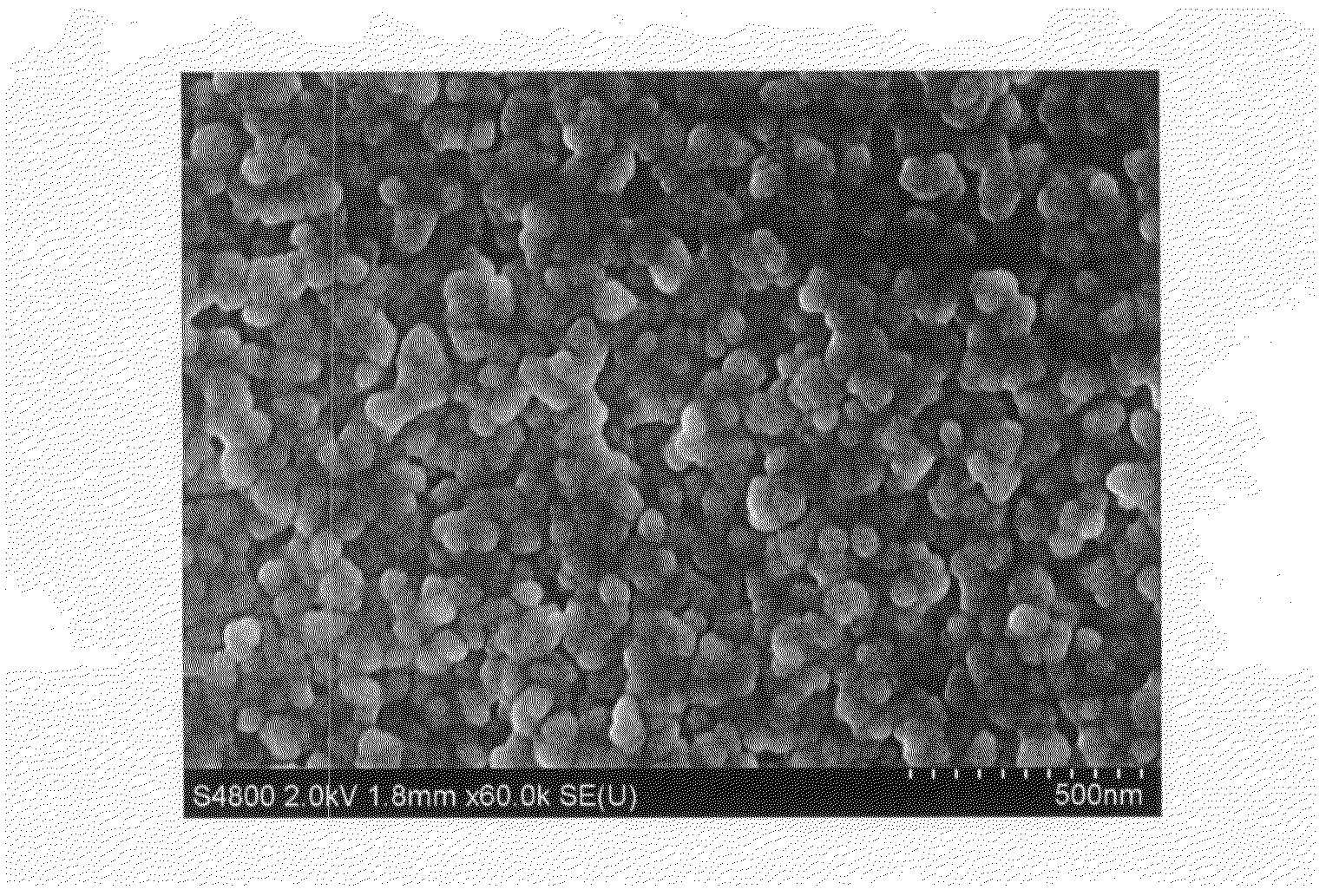Aqueous pigment dispersion and applications thereof
a pigment and dispersion technology, applied in the field of aqueous pigment dispersion, can solve the problems of hardly proceeding in general polymerization, high cost of polymer, and unable to meet the requirements of aqueous pigment, and achieves the effects of high microdispersibility, high dispersion stability, and easy dissolution in water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
Synthesis of High-Molecular Dispersant-1
[0135]To a reactor composed of a 1-L separable flask fitted with a stirrer, reflux condenser, thermometer and nitrogen inlet tube, diethylene glycol dimethyl ether (hereinafter called “diglyme”) (241.5 parts), 2-iodo-2-cyanopropane (hereinafter called “CP-1”) (6.2 parts), methyl methacrylate (hereinafter abbreviated as “MMA”) (180 parts), acrylic acid (hereinafter abbreviated as “AA”) (14.4 parts), azobisisobutyronitrile (hereinafter abbreviated as “AIBN”) (5.2 parts), and iodosuccinimide (hereinafter abbreviated as “NIS”) (0.1 parts) were added, followed by stirring while allowing nitrogen to flow. The reaction temperature was raised to 75° C., at which polymerization was conducted for 3 hours.
[0136]Three hours later, a portion of the polymerization mixture was sampled, and its solid content was measured. As a result, the solid content was found to be 42.0%, and therefore, the monomers were confirmed to be polymerized substantially in their e...
synthesis examples 2 to 13
[0146]Solutions of block-polymer-type high-molecular dispersants-2 to -13 were prepared as in Synthesis Example 1, and are summarized in Tables 1 to 4. CP-1 was commonly used as an initiating compound. The use amount of CP-1 in each of Synthesis Examples 2 to 10 was the same as that in Synthesis Example 1, while the use amount of CP-1 in each of Synthesis Examples 11 to 13 was a half of its use amount in Synthesis Examples 2 to 10. When neutralized, the amount of the aqueous alkaline solution was a half of the amount of the organic solvent used in the polymerization. The abbreviations in Tables 1 to 4 have the following meanings.[0147](1) DPDM: dipropylene glycol dimethyl ether[0148](2) Diglyme: diethylene glycol dimethyl ether[0149](3) MMA: methyl methacrylate[0150](4) AIBN: azobisisobutyronitrile[0151](5) NIS: iodosuccinimide[0152](6) BzMA: benzyl methacrylate[0153](7) SI: succinimide[0154](8) IA: 2-t-butyl-4,6-dimethylphenol[0155](9) V-65: azobis(dimethylisovaleronitrile)[0156](1...
synthesis example 14
Synthesis of High-Molecular Dispersant-14
[0165]Using a similar reactor as in Synthesis Example 1, diglyme (227 parts), CP-1 (6.2 parts), MMA (84 parts), AA (13.7 parts), AIBN (5.2 parts), and NIS (0.45 parts) were combined, followed by stirring while allowing nitrogen to flow. The reaction temperature was raised to 75° C., at which polymerization was conducted for 3 hours. Three hours later, a portion of the polymerization mixture was sampled, and its solid content was measured. As a result, the solid content was found to be 19.2%, and therefore, the monomers were confirmed to be polymerized substantially in their entirety. Further, the molecular weight was measured by GPC. As a result, Mn was found to be 2,300, and PDI was found to be 1.38. The acid value of the polymer was 104.2 mgKOH / g.
[0166]To the polymerization system, a mixture of BzMA (70.4 parts) and AIBN (0.7 parts) was added, followed by polymerization at the same temperature for 3 hours. When its solid content was measure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com