Treatment of microbial infections
a technology for microbial infections and infections, applied in the field of microbial infections, can solve the problems of increasing multi-drug resistance (mdr), difficult to eradicate rapid progressive and highly destructive joint diseases, and serious medical problems of hematogenous acquired bacterial arthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
rClfA A Region Truncates Comprising N1, N2 and N3 (Amino Acids 40-559)
Material and Methods
[0144]Full details of the numeric references in brackets given in the Examples are provided at the end of this section.
Mice
[0145]NMRI mice were obtained from Scanbur BK (Sollentuna, Sweden) and were maintained in the animal facility of the Department of Rheumatology, University of Göteborg, Sweden. Göteborg animal experiment ethical board approved the experiments. They were housed up to 10 animals per cage with a 12 h light-dark cycle, and were fed standard laboratory chow and water ad libitum. The animals were 6 to 16 weeks old at the start of the experiments.
Bacterial Strains
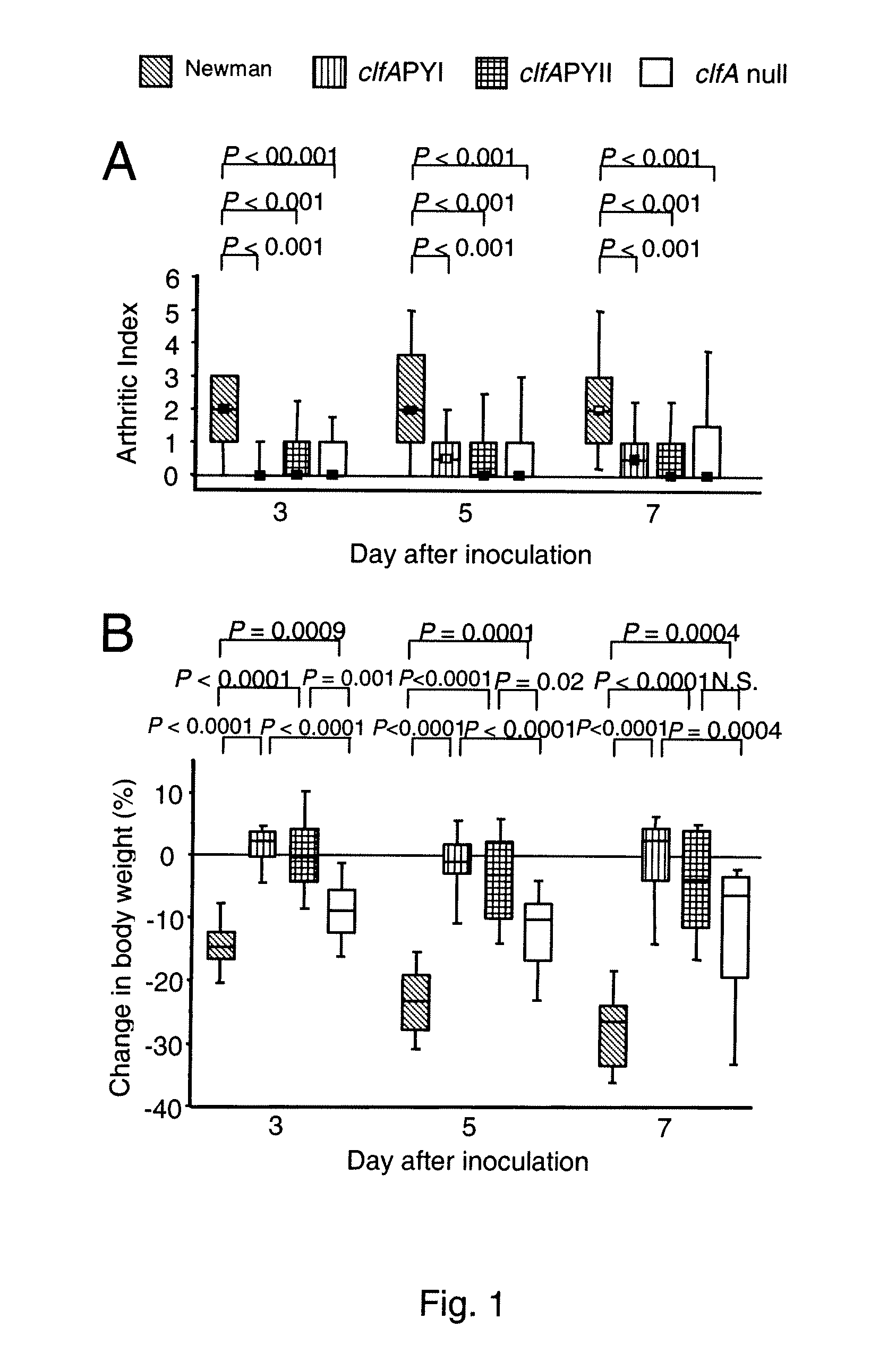
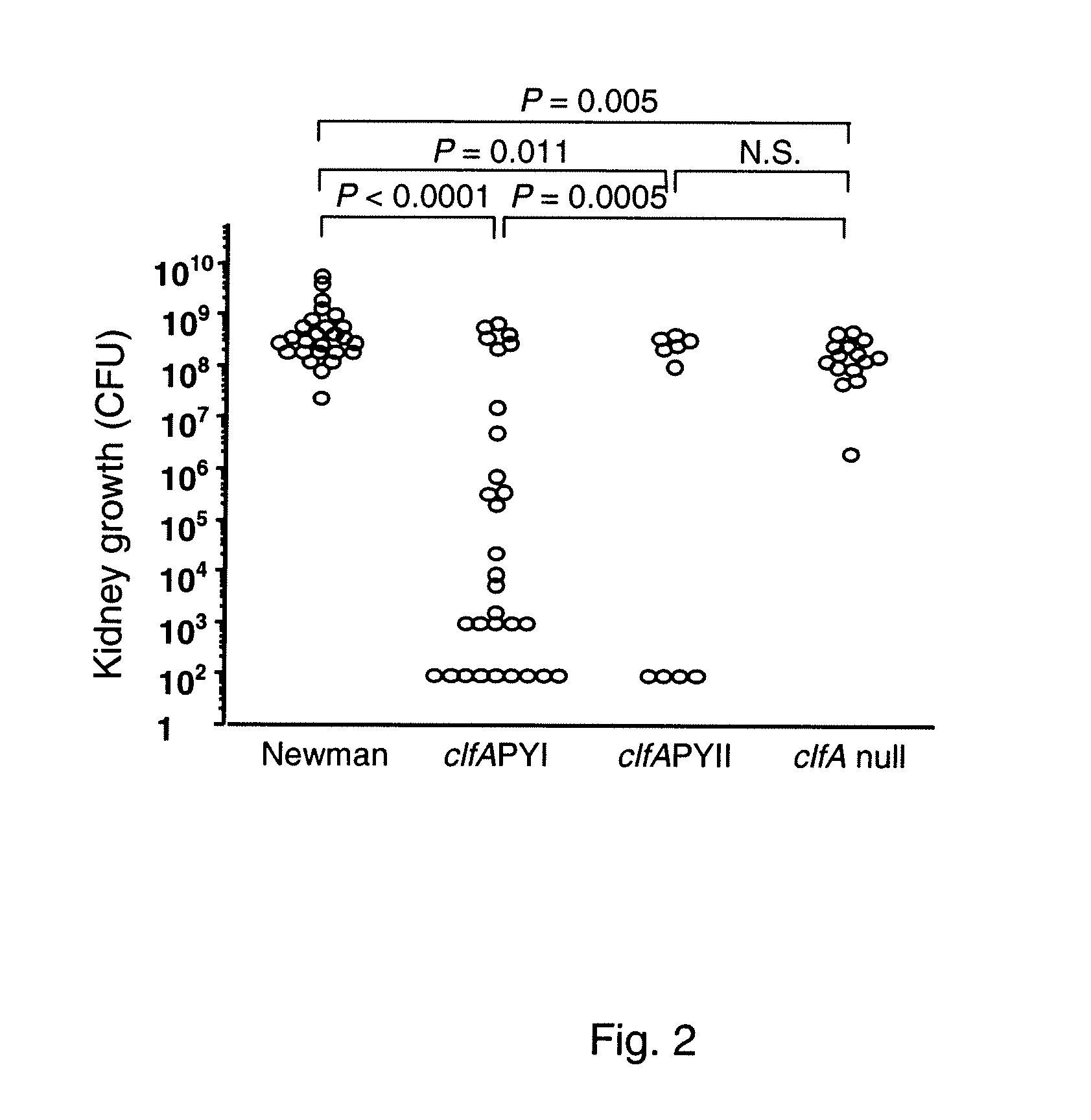
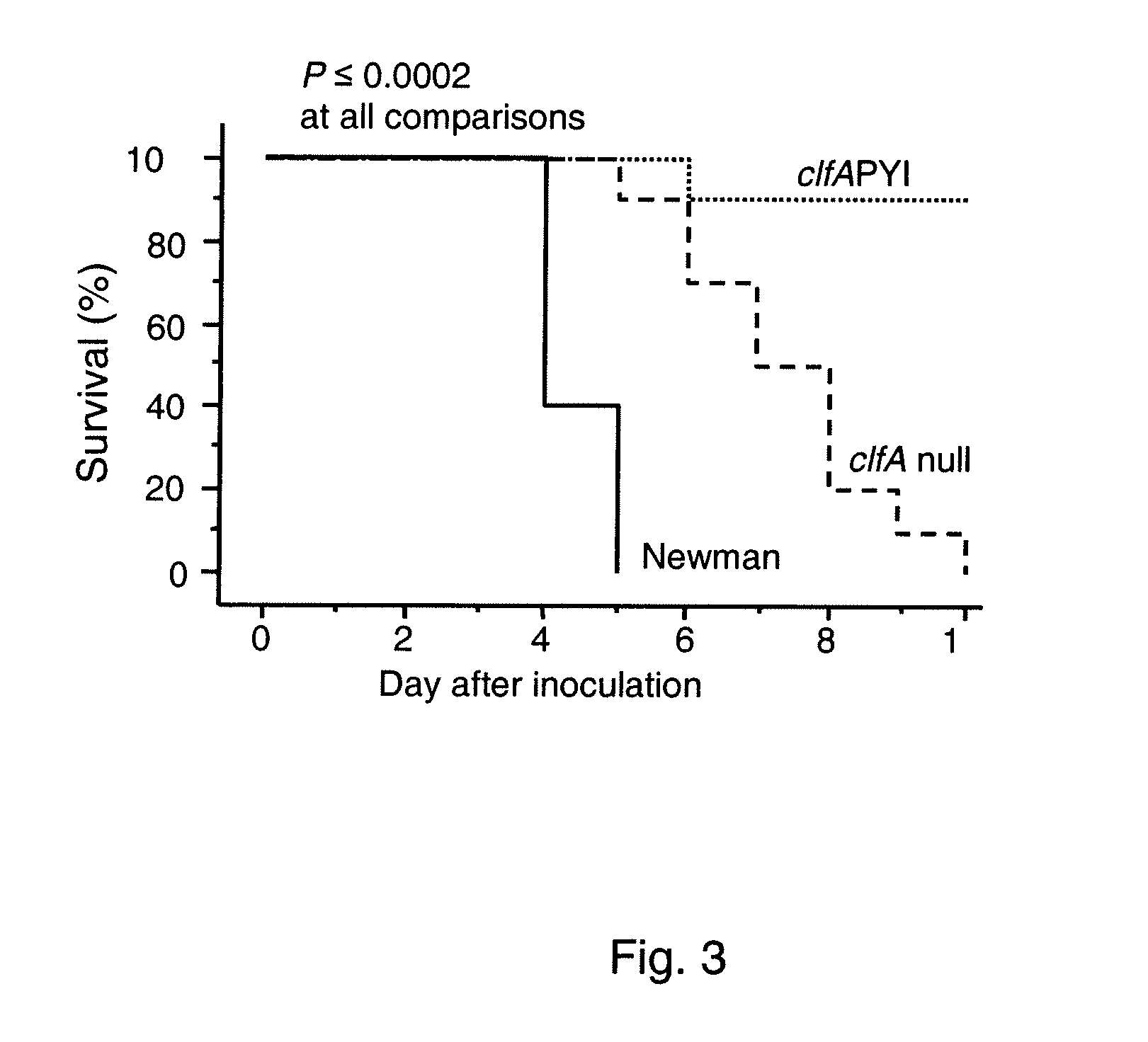
[0146]For infection of animals the S. aureus wildtype strains Newman (14) and LS-1 (11) and constructed derivatives thereof were used. The clfA P336SY338A (clfAPYI) and clfA P336AY338S (clfAPYII) derivatives were constructed in strain Newman and transduced to strain LS-1 (see below). The deletion mutants Newman clfA2::Tn9...
example 2
rClfA A Region Truncate Comprising N2 and N3 (rClfA 221-559)
Materials & Methods:
[0198]The protocols outlined in Example 1 were followed in this example which utilized[0199]rClfA 221-559 (i.e. ClfA A region truncate comprising N2 and N3 corresponding to amino acids 220-559)[0200]rClfAPY221-559; and[0201]BSA.
[0202]There were 15 female NMRI mice per group who were 8 weeks old at start of experiments. In this Example, the constructs used for immunization were ClfA wild type / native N2N3 truncate, ClfA N2N3 truncate with mutation PY as defined in Example 1. BSA was used as the control.
Vaccination with Wild-Type and Mutant Recombinant ClfA
[0203]The mice were immunized with rClfA 221-559, rClfAPY 221-559 or BSA in accordance with the protocol of Example 1.
[0204]Purified rClfA221-559, rClfAPY221-559 (i.e. ClfAPYI recombinant protein A subdomains N2 and N3) or BSA were dissolved in PBS and emulsified 1:1 in Freund's complete adjuvant. Two hundred μl of the emulsion containing 30 μg (=0.79 nmo...
example 3
ClfA A Region Truncate (δ / Delta Latch Truncate)
Materials & Methods:
[0210]The protocols outlined in Example 1 were followed in this example which utilized the following construct:[0211]rClfA 221-531 (i.e. rClfA A region truncate comprising N2 and N3 amino acids 220-559 but without the latching peptide amino acids 532-538 and the subsequent proline-rich residues.
[0212]There were 15 female NMRI mice in the group who were 8 weeks old at start of experiment. In this Example, the above construct was used for immunization. The mice were immunized with the above truncate in accordance with the protocol of Example 1.
Vaccination with Wild-Type and Mutant Recombinant ClfA
[0213]Purified rClfA221-531 was dissolved in PBS and emulsified 1:1 in Freund's complete adjuvant. Two hundred μl of the emulsion containing 0.79 nmol of protein was injected s.c. on day 0. First booster immunization with 0.79 nmol of protein in physiologic saline in incomplete Freund's adjuvant was performed on day 12. Second...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Immunogenicity | aaaaa | aaaaa |
| Hydrophobicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com