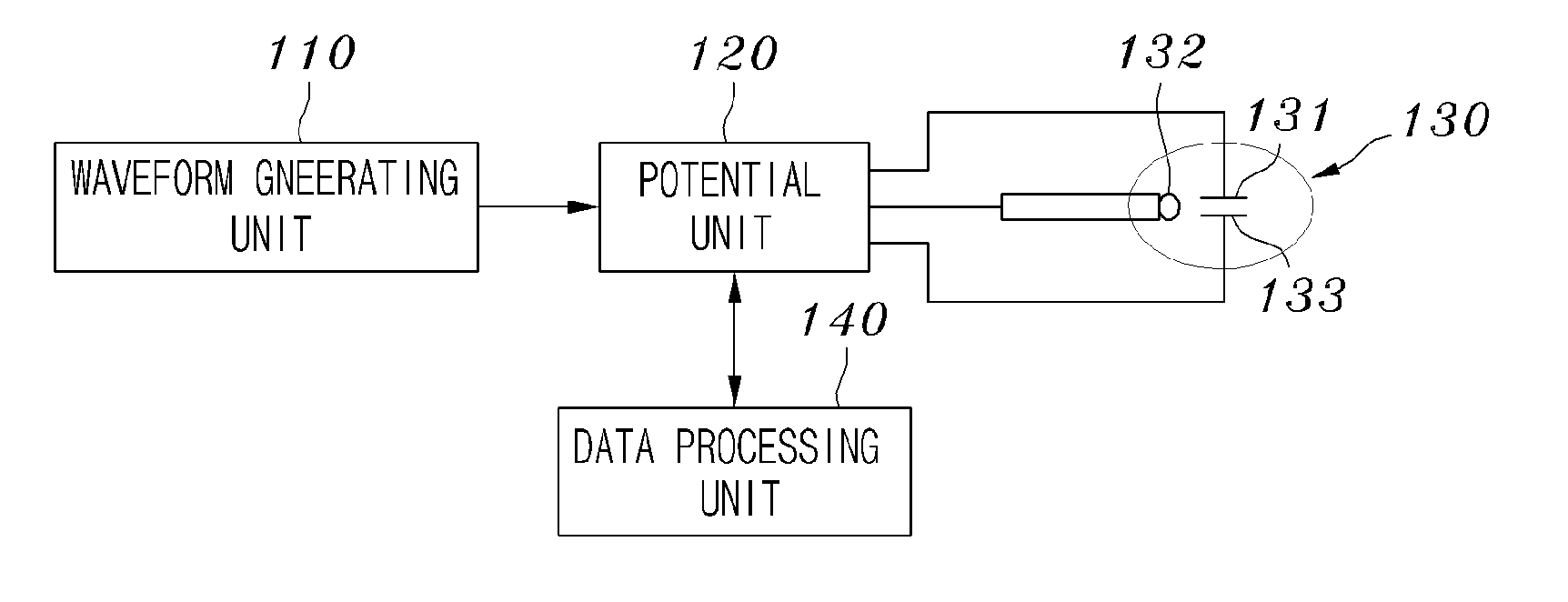
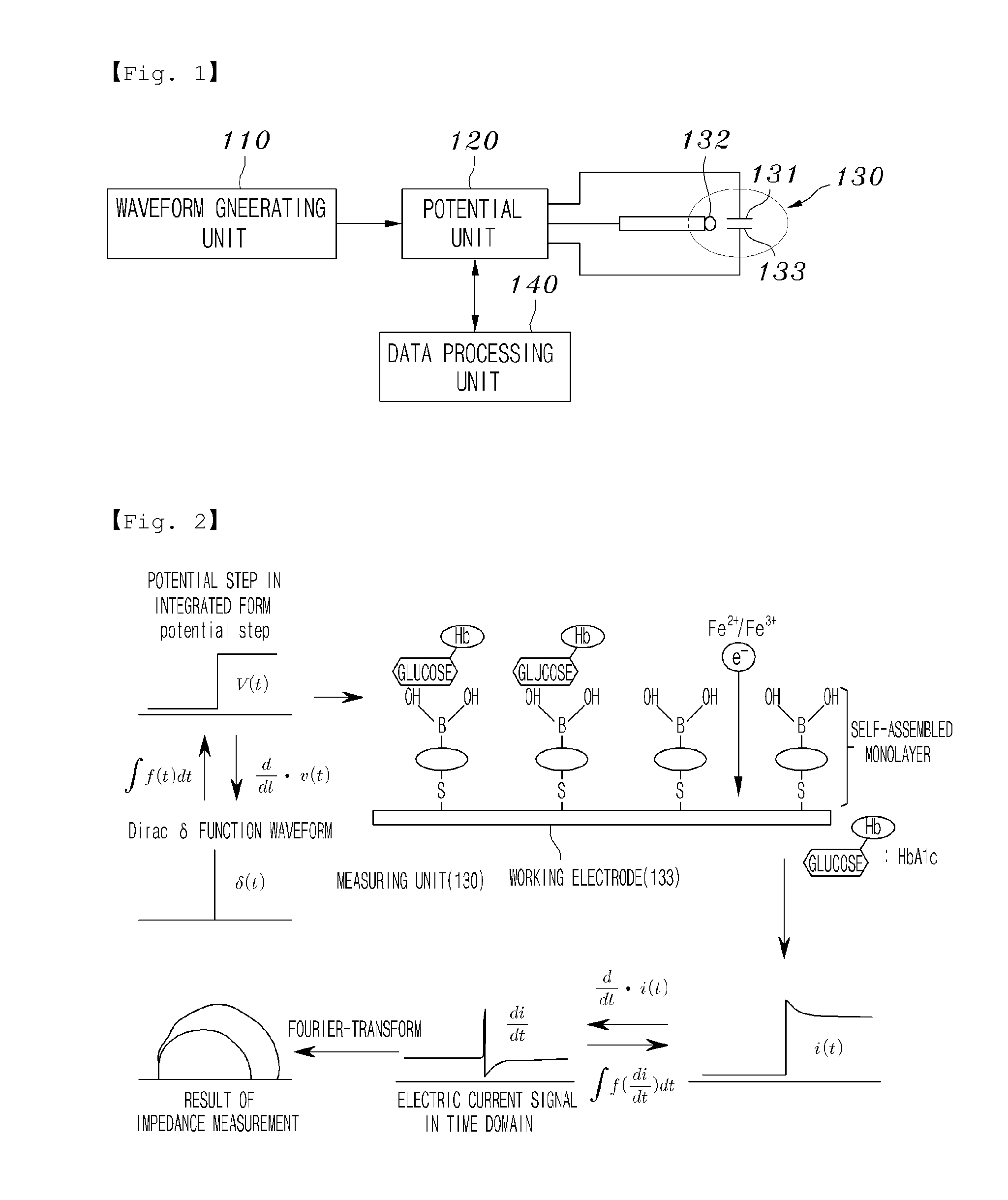
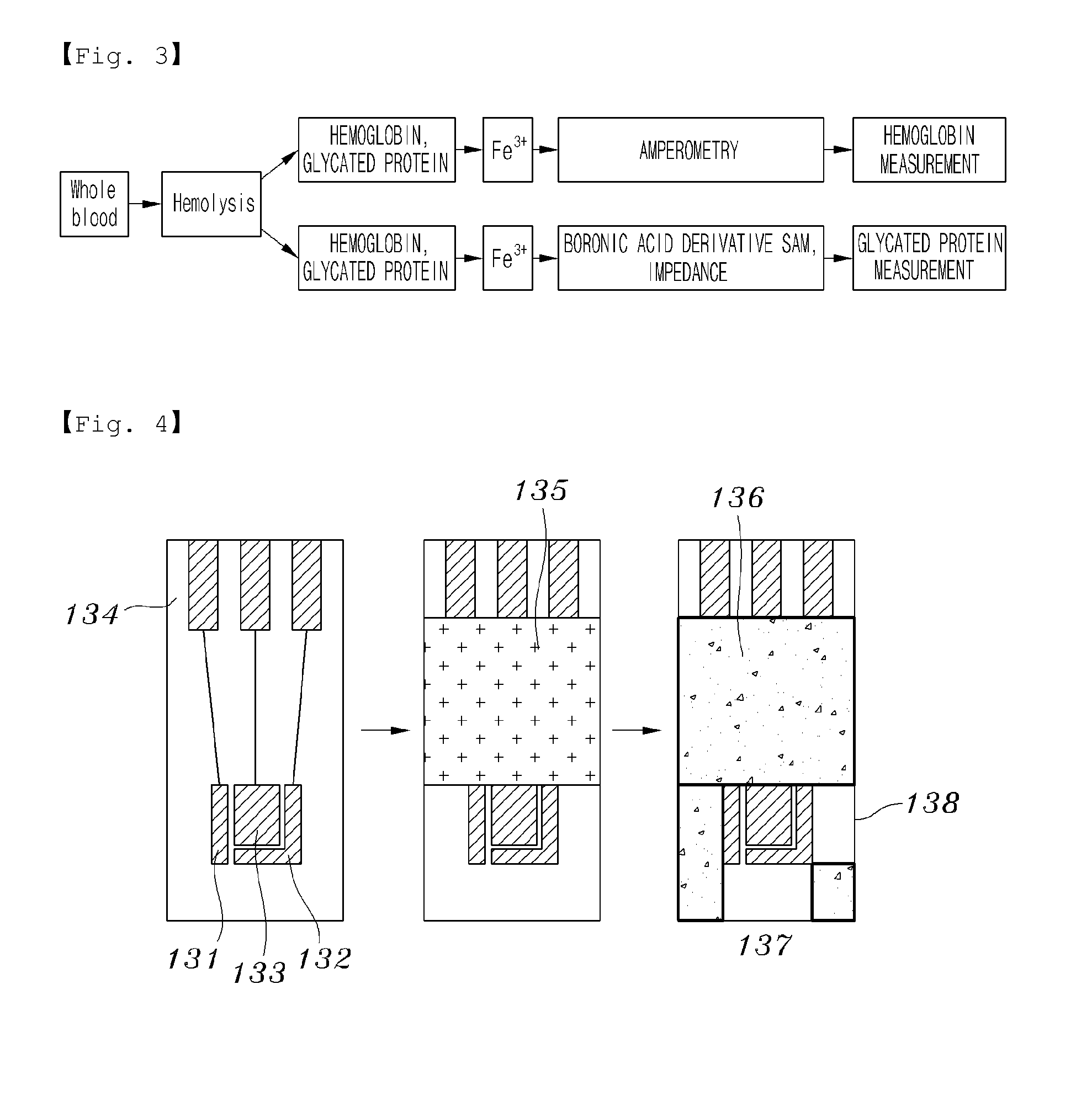
Device for measuring proteins using biosensor
a biosensor and protein technology, applied in the direction of instruments, material electrochemical variables, material impedance, etc., can solve the problems of difficult reproducibility, difficult to achieve consistent quality of sensors, and complex measurement of glycosylated protein concentration, etc., to achieve accurate measurement, shorten measurement time, and reduce the effect of tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
Measurement of Glycated Protein Based on Impedance Using Fourier Transform
The experiment is based on the method and principle explained above, with the following conditions of the experiment. The buffer solution used blank solution of pH 7.4, 10 mM PBS and 2.5 mM Fe3+, hemolysis blood sample was used, and and glycated hemoblogin was used in a concentration of 4.5%, 5.2%, 7.0%, 9.2% and 11.6%. The SAM was formed on the gold working electrode (133) using 10 mM thiophene boronic acid.
The graphical representation of sensitivity of FIG. 7 indicates the result of measurement in which randomized controlled trial (Rct) was measured according to each concentration and represented in the form of ratio of the blank solution with respect to charge transfer resistance. FIG. 7 represents the measurement of impedance using frequency response analyzer (FRA). The conventional method requires long measurement time and long data processing time to measure the average signal to improve signal-to-noise ...
embodiment 2
applied the same method explained above in Embodiment 1, and the graphical representation of sensitivity and the calibration line of FIGS. 9 and 10 respectively represent the result of measurement.
Referring to FIGS. 9 and 10, the linear increment of impedance in accordance with the increase of concentration of glycated protein confirms the suitability of a device according to the present invention as a biosensor using impedance and also confirms that glycated protein is effectively detected by the impedance method using Fourier transformation.
embodiment 3
Measurement of Glycated Hemoglobin Using Fourier-Transformed Impedance
The method using Fourier-transformed impedance was applied to the electrode of Embodiment 1 by using a potentiostat which has the potential increasing time shorter than 50 ms. Impedance data was collected for the first 2.5 seconds by using 10 mv potential step corresponding to 0.4 Hz. From the data obtained from chronoamperometry, impedance data was computed within a range of 0.4-10 kHz, and cyclic voltammetry was conducted at 400 mV / s scan speed for 2.5 seconds. Stock solution in various concentrations was prepared from 4 mL pH 8.5 buffer solution, and 40 μL was taken from each stock solution and injected into solution containing electrode and oxidation-reduction pair to measure the charge transfer resistance. As for standard substance, JCCLS CRM004a (Japanese Committee for Clinical laboratory Standards) containing 4-13% of glycated hemoglobin with respect to the total amount of hemoglobin (140±10 g / L) was used. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com