Granulate for the formulation of orodispersible tablets
a technology of granulate and orodispersible tablets, which is applied in the direction of biocide, plant growth regulators, pharmaceutical non-active ingredients, etc., can solve the problems of increasing the mass of the tablet restrictor, slowing down the disintegration, and the disintegration function of the disintegrant, so as to achieve less pressure, good mechanical properties, and greater porosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
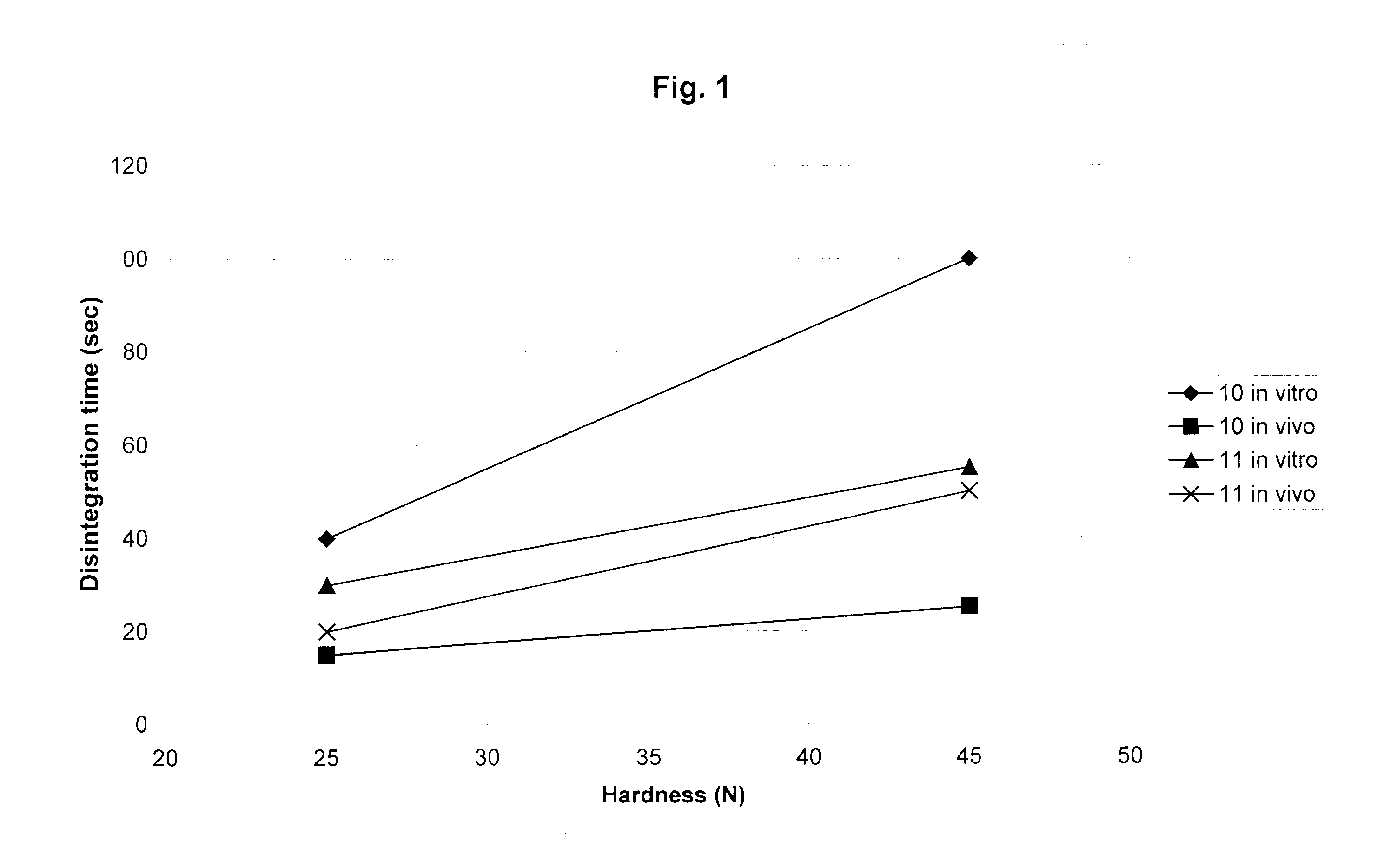
Image
Examples
example 1
[0077]A mixture of powdered mannitol (average size less than 100 μm) and powdered sorbitol (average size between 200 μm and 250 μm) in a weight ratio of 9:1 was used to prepare the granulate using different granulation methods.
[0078]Using the dry granulation technique, the mixture was first compacted by a tablet press into slugs having a diameter of approximately 20-30 mm. Using different compression forces, slugs TA, TB, TC having respective hardness of 20N, 50N and 120N were obtained. The slugs were then broken up using an oscillating granulator and sieved through a 1000 μm sieve. Granulates A, B and C obtained from slugs TA, TB, TC respectively had the average sizes and densities indicated in Table 1.
[0079]Using the wet granulation technique, the mixture was wet granulated with purified water in an Erweka AR400 granulator. The paste was dried on a fluidised bed and sieved through a 1000 μm mesh. Granulate D had an average size and density as indicated in Table 1 below.
[0080]Using...
example 2
[0083]Granulate E according to this invention was compared with a series of commercial excipients ready for compression. The comparison was made by comparing a series of tablets as described in Example 1 and measuring the resulting hardness of each tablet obtained. The results are summarised in Table 3 below.
TABLE 3ExcipientHardness (N)CIGranulate E3004.62Xylitab 200 (Danisco)2854.38Sorbitol (Roquette)2453.77Maltodextrin2153.31Isomalt (Diamalt)2143.29Emdex (Mendell)2123.26Ercawax 4000 (Erca)1963.02Pearlitol (Roquette)1632.51Lactose DC1051.62Microtal (T&L)921.42Saccharose751.15Fructose500.77Citric acid450.69
[0084]The data in Table 3 showed that granulate E has the best compressibility index (CI) in comparison with the excipients known in the art.
example 3
[0085]Granulate E according to this invention was prepared following the procedure described in Example 1, varying the process conditions (temperature and humidity) of the air. The results are summarised in Table 4 below.
TABLE 4Temperature (° C.)Relative humidity (%)Result800.1Non-conforming product -product melted and turnedto yellow700.1Optimum product -moisture content 0.1% after20 minutes drying500.1Non-conforming product -product moisture content0.25% after 50 minutesdrying (long)701Conforming product -product moisture content0.20% after 60 minutesdrying (long)7010Conforming product -product moisture content0.20% after 70 minutesdrying (long)7025Non-conforming product -product moisture content0.25% after 70 minutesdrying (long)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com