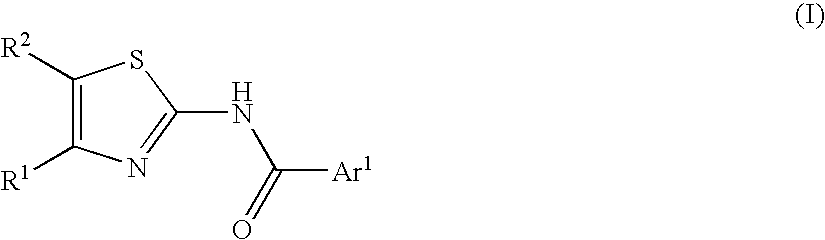2-acylaminothiazole derivative or salt thereof
a technology of acylaminothiazole and derivative, applied in the field of new acylaminothiazole derivative, can solve the problems of insufficient amount of platelets, difficult to sufficiently improve thrombocytepenia, and inability to provide sufficient platelets, etc., and achieve the effect of increasing the number of platelets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
[0225]To a solution of 4.18 g of 4-chloro-2-acetylthiophene in 30 ml of diethylether, 1.5 ml of bromine was added under ice cooling, and the mixture was stirred at room temperature for 2 hours. Water was added to the reaction solution, and the organic phase was extracted. The obtained organic layer was washed with brine and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to obtain brominated compound. To a solution of the brominated compound in 30 ml of EtOH, 2.1 g of thiourea was added at room temperature, and the mixture was stirred at 80° C. overnight. The precipitate was filtered, and obtained solution was evaporated under reduced pressure. Chloroform was added and then an organic layer was washed with aqueous potassium carbonate and brine, and dried over sodium sulfate. The residue obtained by the evaporation of the solvent under reduced pressure was washed with hexane:EtOAc=1:1 to obtain 2.57 g of 2-amino-4-(4-chlorothiophen-2-yl)thiazole...
reference example 9
[0232]To a solution of 6.0 g of 2-amino-4-(4-fluorophenyl)thiazole in 100 ml of THF, 1.60 ml of bromine was added dropwisely, and the mixture was stirred at room temperature for 90 minutes. After evaporation of the solvent, 100 ml of DMF, 10.4 g of 1-cyclohexylpiperazine, and 17.2 ml of triethylamine were added, and the mixture was stirred at 90° C. for 31 hours. The solvent was evaporated under reduced pressure, and the residue was mixed with saturated aqueous NaHCO3 and extracted with chloroform. The organic layer was washed with brine, and dried over sodium sulfate. The residue obtained by the evaporation of the solvent under reduced pressure was purified by silica gel column chromatography (elute: chloroform-MeOH=100:1·100:3) to obtain 11.26 g of 2-amino-5-(4-cyclohexylpiperazin-1-yl)-4-(4-fluorophenyl)thiazole.
[0233]Compounds of Reference Examples 10-40 as shown in Table 4 were synthesized employing each corresponding starting material, in the same manner as described in Refere...
reference example 41
[0234]To a solution of 0.5 g of the compound of Reference Example 1 in 5 ml of DMF, 0.45 g of N-bromosuccinimide was added under ice cooling, and the mixture was stirred at the same temperature for 50 minutes. To the reaction mixture, 0.6 g of cyclohexylpiperazine and 0.6 ml of triethylamine were sequentially added, and the mixture was stirred at 70° C. for 3 days. The solvent was evaporated under reduced pressure, chloroform was added to the residue, and then the organic layer was washed with aqueous potassium carbonate and brine, and dried over sodium sulfate. The residue obtained by the evaporation of the solvent under reduced pressure was purified by silica gel column chromatography (elute: hexane-EtOAc=1:1) to obtain 300 mg of 2-amino-4-(4-chlorothiophen-2-yl)-5-(4-cyclohexylpiperazin-1-yl)thiazole.
[0235]Compounds of Reference Examples 42-71 as shown in Table 4 were synthesized employing each corresponding starting material, in the same manner as described in Reference Example ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com