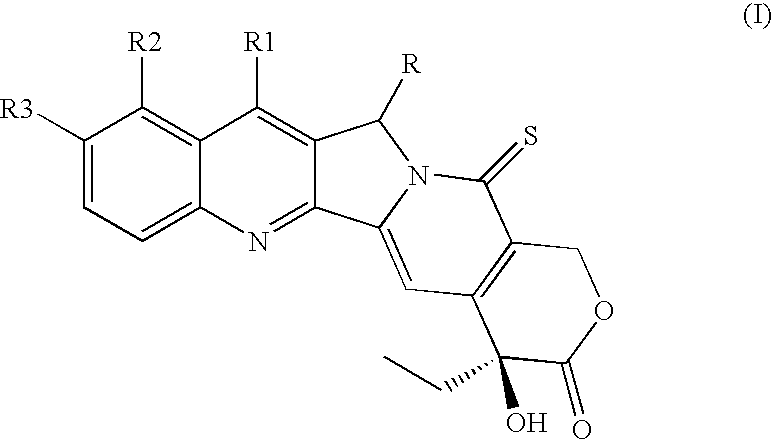
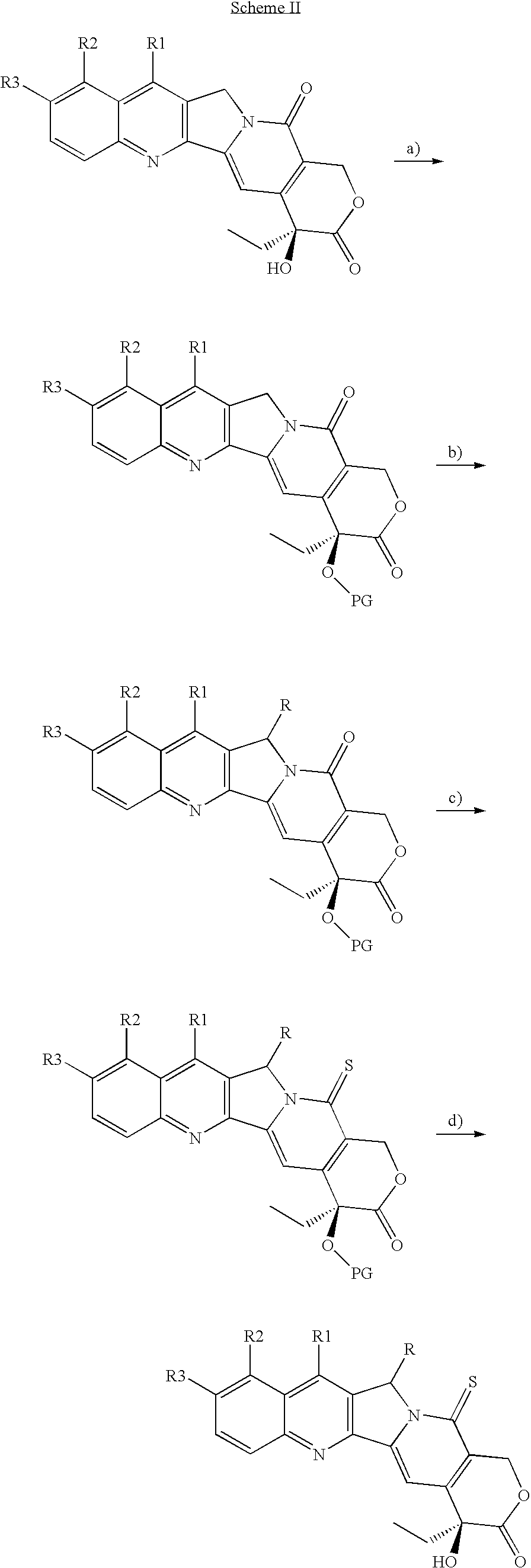
Camptothecin derivatives with antitumor activity
a technology of antitumor activity and derivatives, which is applied in the direction of heterocyclic compound active ingredients, biocides, drug compositions, etc., can solve the problems of hematological toxicity of lurtotecan, product accumulation in the body, and poor tumor affecting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
20-OTES-camptothecin
[0047]Camptothecin (0.100 g, 0.287 mmols) is suspended in anhydrous dimethylformamide (3 mL), under inert atmosphere, and the resulting suspension is added with imidazole (0.980 g, 1.44 mmols). The mixture is stirred for 10′ minutes, subsequently triethylsilyl chloride (TES-Cl) (0.193 mL, 1.15 mmols) is dropped therein, followed by addition of 4-dimethylamino pyridine (DMAP) (0.040 g 0.287 mmols). After 46 h, the reaction mixture is evaporated under vacuum, (TLC control of the complete disappearance of the reagent, eluent CH2Cl2 / MeOH=30 / 1). The solid is subsequently redissolved in CH2Cl2 and washed with H2O and saturated NH4Cl. The aqueous phase is extracted with CH2Cl2 (2×10 mL). The organic phases are combined and dried over Na2SO4, filtered and concentrated under vacuum, thereby obtaining the desired product (0.133 g, 0.287 mmols) as a pale yellow solid.
[0048]1H NMR (CDCl3, 400 MHz) δ 8.37 (s, 1H, Ar, H-7), 8.25 (d, 1H, J=8.4 Hz, Ar), 7.92 (d, 1H, J=8.0 Hz, Ar...
example ii
20-OTES-Thio-camptothecin
[0049]Camptothecin 20-OTES (0.664 g, 1.44 mmols), is dissolved in anhydrous xylene (20 mL) with stirring under inert atmosphere. Subsequently Lawesson's reagent (LR), (0.523 g, 1.29 mmols) is added and the reaction is heated to 90° C. The reaction mixture is reacted for 18 h at 90° C., monitoring by TLC the disappearance of the reagent (Hexane / AcOEt=1 / 1). The solvent is evaporated off under vacuum and the residue is purified by flash chromatography (SiO2, Hexane / AcOEt=4 / 1 then 7 / 2), thereby obtaining the desired product (0.578 g, 1.21 mmol, 84%) as an intense yellow solid.
[0050]1H NMR (CDCl3, 400 MHz) δ 8.46 (s, 1H, Ar, H-7) 8.29 (d, 1H, J=8.4 Hz, Ar), 8.03 (s, 1H, H-14), 7.97 (d, 1H, J=8.4 Hz, Ar), 7.86 (t, 1H, J=21.8 Hz, Ar), 7.69 (t, 1H, J=8.4 Hz, Ar), 6.15 (d, 1H, J=16.9 Hz, H-17), 5.62 (d, 1H, J=21.0 Hz, H-5), 5.57 (d, 1H, J=21.0 Hz, H-5), 5.34 (d, 1H, J=16.9 Hz, H-17), 1.94 (d, 1H, J=7.6 Hz, H-19), 1.90 (d, 1H, J=7.6 Hz, H-19), 1.05-0.91 (m, 12H), 0.82...
example iii
Preparation of Thio-camptothecin (IDN 6070)
[0051]20-OTES Thio-camptothecin (0.150 g, 0.314 mmols) is dissolved in anhydrous THF (10 mL) with stirring under inert atmosphere, subsequently Et3.N3HF (0.140 mL, 0.816 mmols) is dropped therein. The reaction mixture is reacted for 48 h at room temperature, monitoring by TLC the disappearance of the reagent (Hexane / AcOEt=1 / 1). The solvent is evaporated off under vacuum and the residue is purified by flash chromatography (SiO2, Hexane / AcOEt=2 / 1 then 1 / 1), thereby obtaining the desired product (0.112 g, 0.307 mmol, 98%) as an intense yellow solid.
[0052]1H NMR (CDCl3, 400 MHz) δ 8.46 (s, 1H, Ar, H-7), 8.27 (d, 1H, J=8.4 Hz, Ar), 8.13 (s, 1H, H-14), 7.97 (d, 1H, J=8.4 Hz, Ar), 7.86 (t, 1H, J=21.8 Hz, Ar), 7.70 (t, 1H, J=8.4 Hz, Ar), 6.25 (d, 1H, J=16.9 Hz, H-17), 5.62 (d, 1H, J=21.0 Hz, H-5), 5.58 (d, 1H, J=21.0 Hz, H-5), 5.37 (d, 1H, J=16.9 Hz, H-17), 3.80 (s, 1H, OH), 1.90 (q, 2H, H-19), 1.03 (t, 3H, J=7.2 Hz, Me). 13C NMR (CDCl3, 100 MHz) δ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com