Vasoconstriction compositions and methods of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
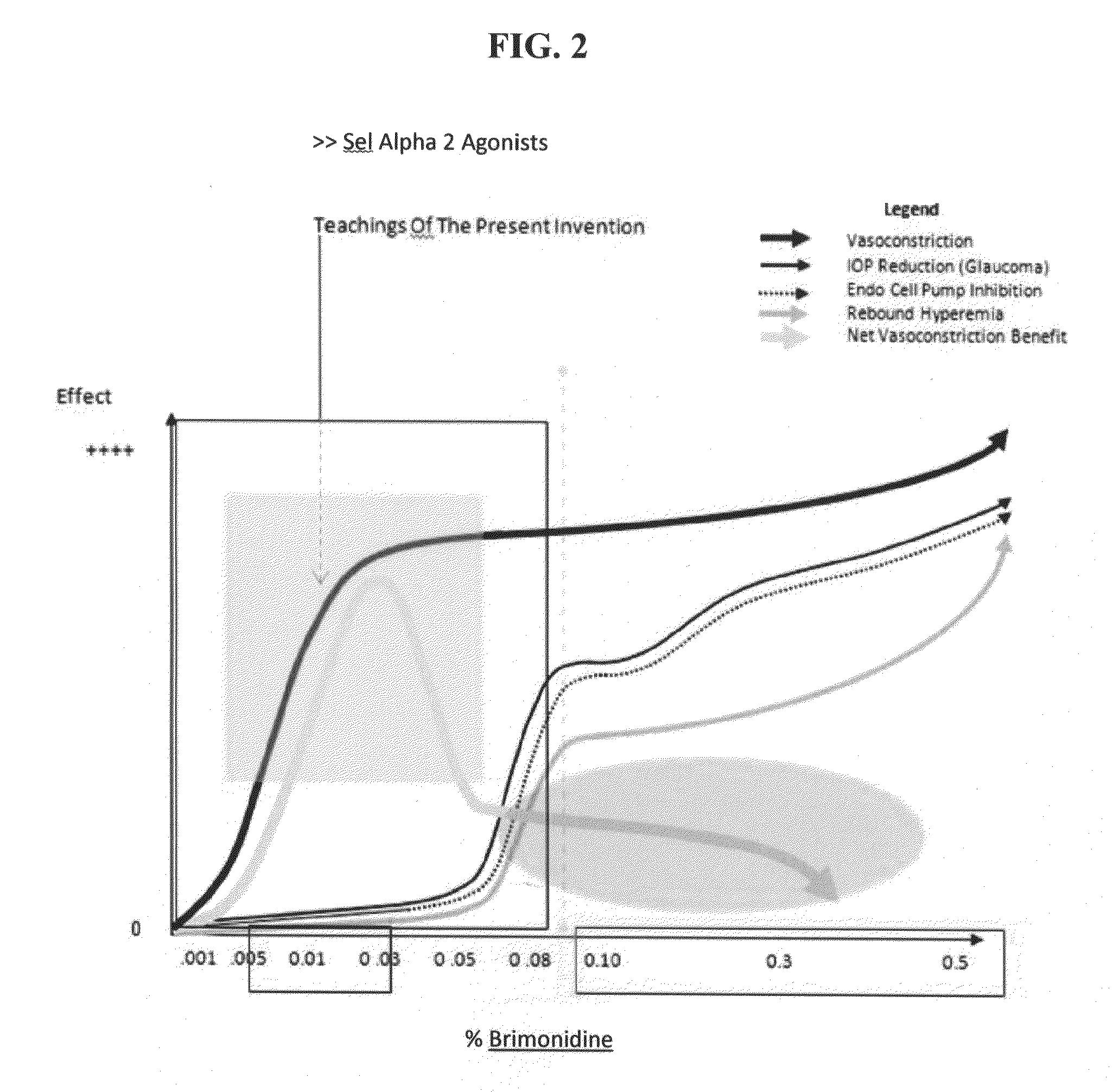
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
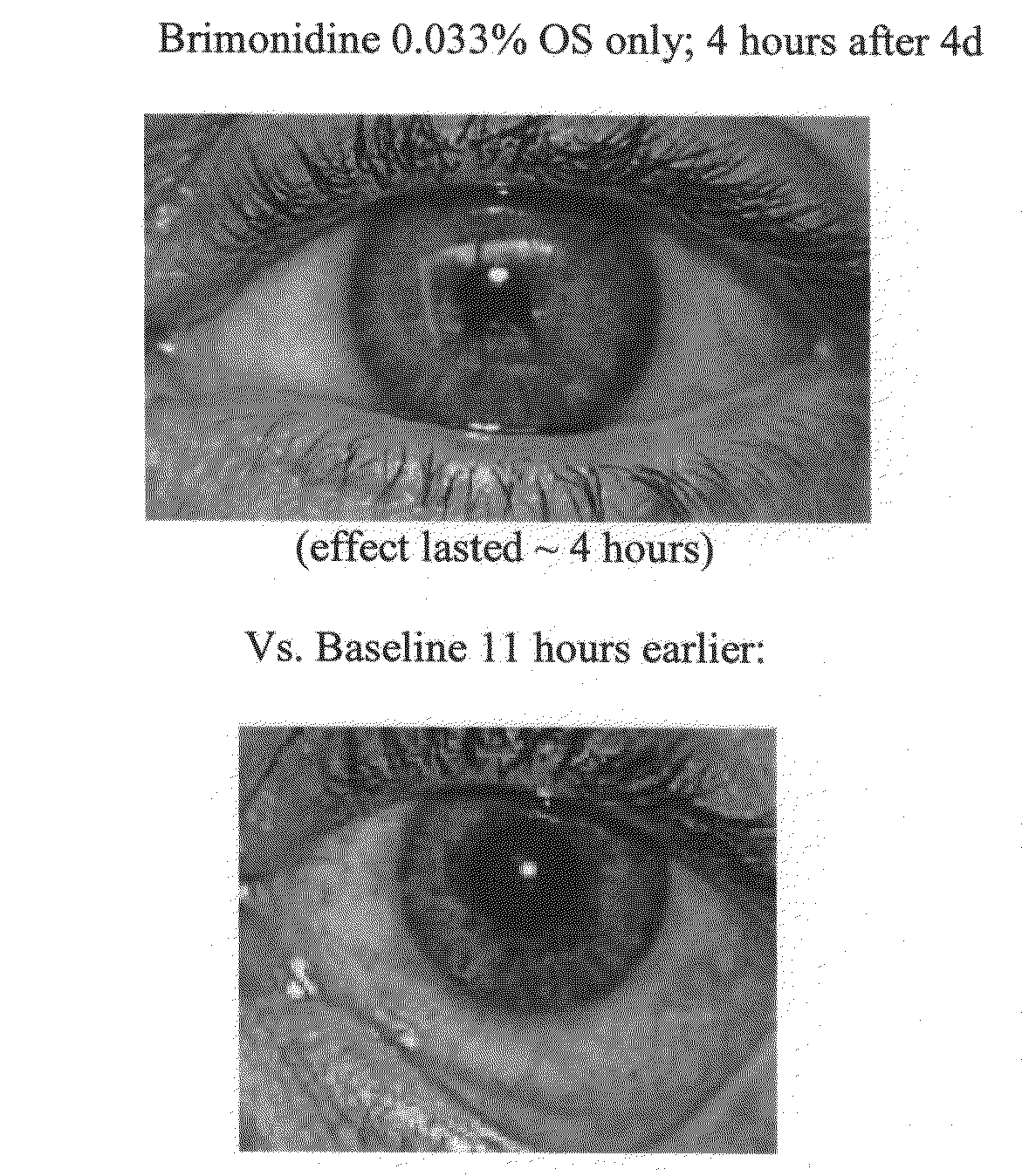
[0180]In this Example, a patient was treated with brimonidine at claimed concentrations and prior art compositions of tetrahydrozoline, oxymetazoline and naphazoline.
[0181]The results clearly demonstrate significant scleral whitening brightening effects of treatment with brimonidine as compared with treatment with prior art compositions.
[0182]The results are shown in FIGS. 4A through 4E.[0183]FIG. 4A shows the base line for both eyes.[0184]FIG. 4B shows a comparison after 180 minutes, where the right eye has been treated with tetrahydrozoline at 0.05% and the left eye was treated with brimonidine at 0.01%[0185]FIG. 4C shows a comparison four hours after baseline (FIG. 4A), where the right eye has been treated with oxymetazoline at 0.025% and the left eye was treated with brimonidine at 0.02%[0186]FIG. 4D shows a comparison where after a further four hours, the right eye has been treated with naphazoline at 0.033%; and the left eye was treated with brimonidine at 0.02%.[0187]FIG. 4E ...
example 2
Lasik Prophylaxis
[0190]Baseline:
[0191]Treatment of 200 patients via the Intralase femtosecond laser with no pretreatment for vasoconstriction—significant postoperative hyperemia and conjunctival hemorrhage with @ 15% petichial or larger hemorrhage when patients were seen postoperative day 1.25% 1+(14) hyperemia first hour +; 50% 2.5+hyperemia first how +; 25% 3+hyperemia first hour +. Flap dislocation rate: <0.1%.
[0192]Treatment Group 1:
[0193]Naphcon-A® (Alcon, Inc; active ingredients: naphazoline hydrochloride 0.25% and pheniramine maleate 0.3%; preserved with benzalkoniurnm chloride) was used on a second group of 50 patients (85 procedures), 12% petichial or larger hemorrhage. 35% 1+hyperemia; 35% 2+hyperemia; 15% 2.5+hyperemia; 15% 3+hyperemia. Some clinical benefit noted. Flap dislocation rate: <0.1%.
[0194]Treatment Group 2:
[0195]Brimonidine 0.2%, used off label, has been reported to cause flap dislocation rates of 5-10% and is currently not indicated nor recommended for this pu...
example 3
[0201]0.03% Brimonidine Nasal Spray: 0.9% saline vehicle used and nasal spray administered to patient with nasal congestion. This was repeated for one week without rebound. Complete relief for 3-5 hours was reached per application for treatment of moderate nasal congestion thought to be allergic in nature. Repeat applications x four hours without rebound. Patient population for this test limited to n of 1.
[0202]The proper dose-response range can be tested with no more than routine experimentation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com