Small Interfering RNA Specific For HCV And Therapeutic Agent For Hepatitis C Comprising The Same
a technology of hcv and therapeutic agent, applied in the field of small interfering rna specific for hcv, can solve the problems of inability to develop new therapies to treat hcv infection, limited efficacy of current therapeutic regimens, and inability to protect infection with vaccines,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
siRNA and siRNA Expression Vector
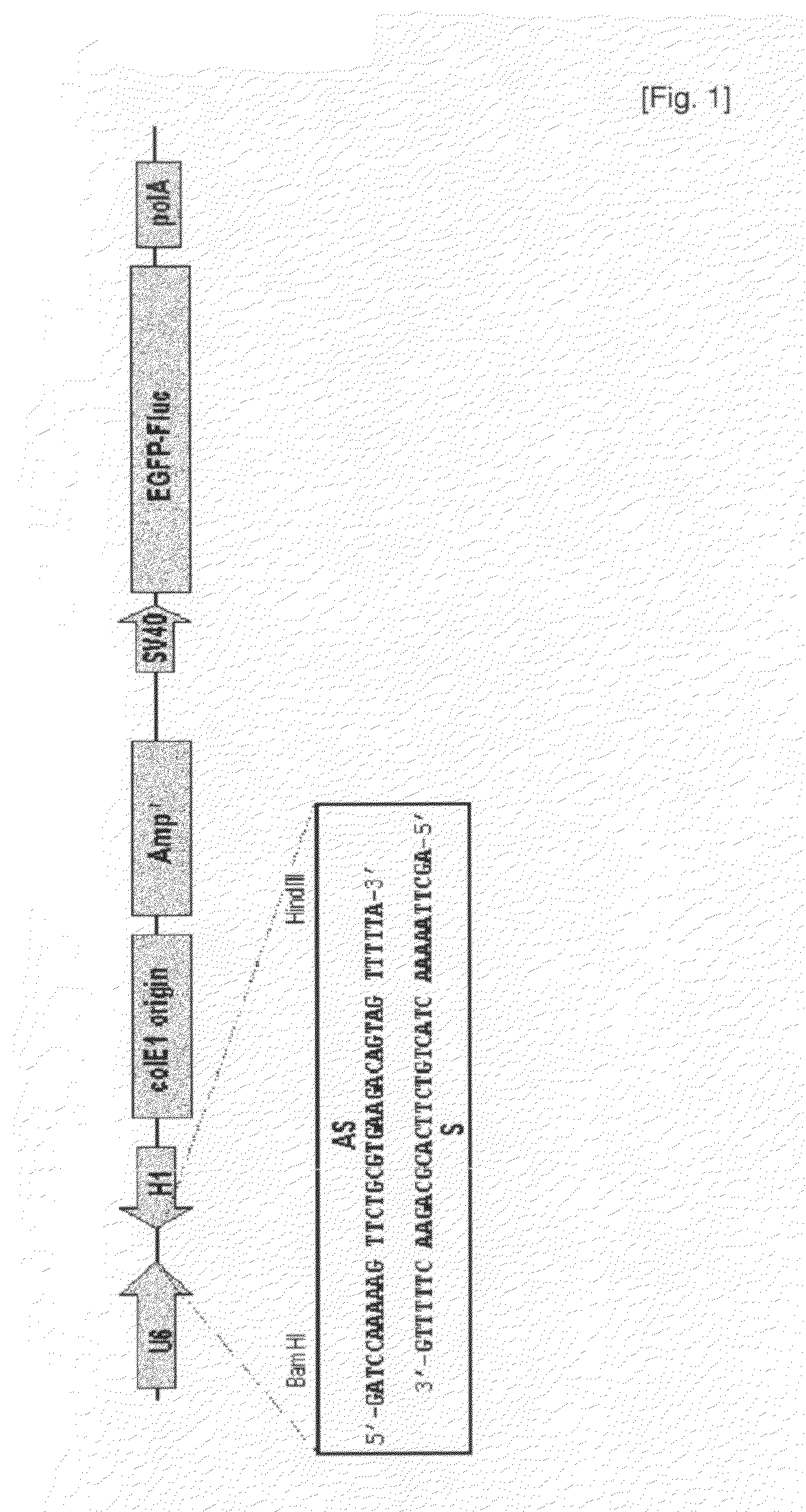
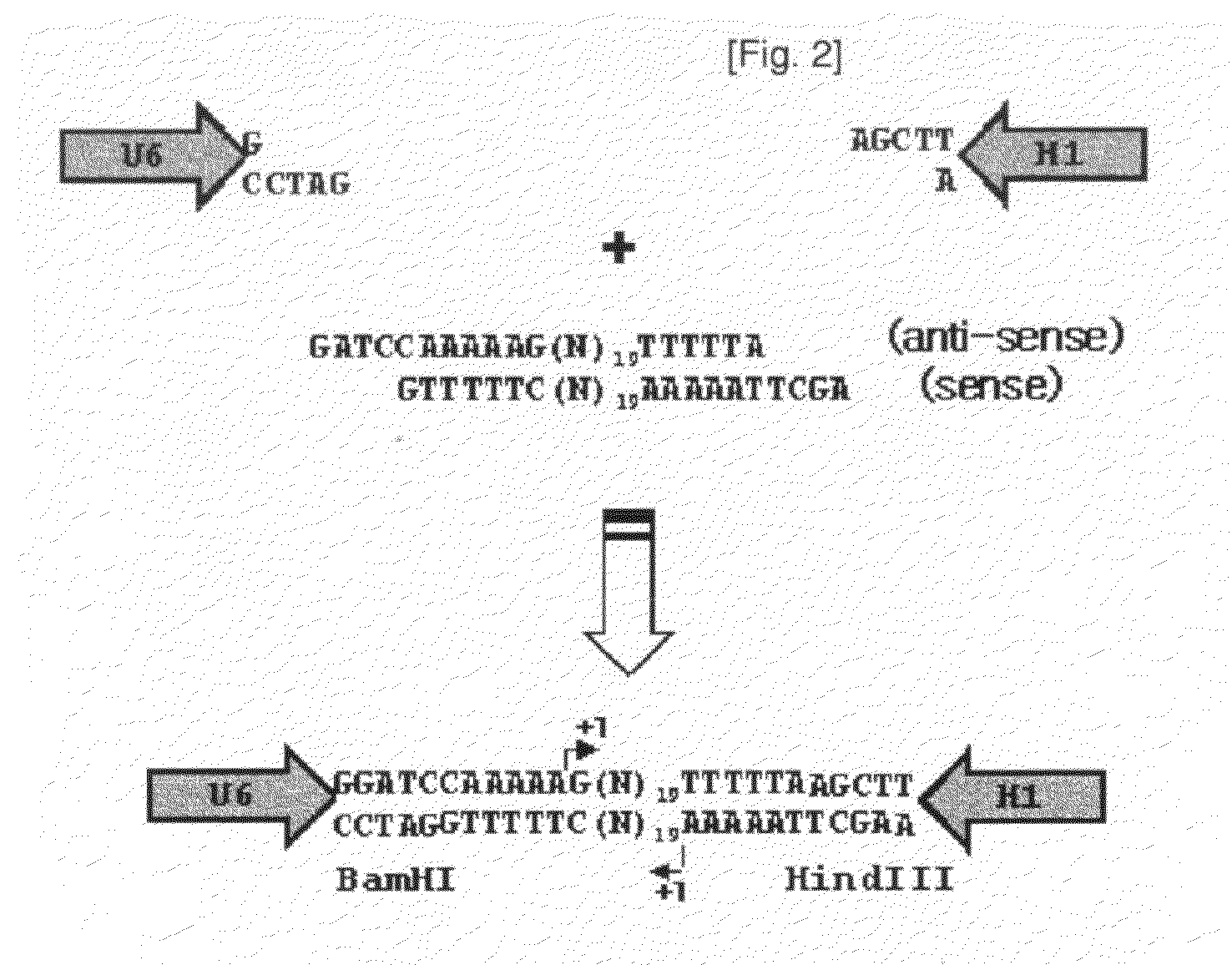
[0052]In mammalian cells, previously siRNA vectors have been designed to transcribe short hairpin RNAs (shRNAs) from an RNA polymerase III promoter (such as U6, H1, and tRNA promoters) or a polymerase II promoter with a poly(A) signal sequence (Brummelkamp et al., Cancer Cell, 2: 243, 2002; Tushcl, Nat. Biotechnol., 20: 446, 2002; Xia et al., Nat. Biotechnol., 20: 1006, 2002). However, shRNA vectors show multiple drawbacks. Their non-natural secondary structure induces that it is hard to synthesize them in bacteria and to sequence, and DNA oligomers to generate them can be costly in the case of high through-put screening. Moreover, it is less facile to generate an siRNA expression cassette containing a promoter to a termination signal without additional sequences for constructing diverse siRNA library. To circumvent these limitations of shRNA expression vectors, the present inventors constructed a vector for direct expression of siRNA, which is trans...
example 2
Inhibition of Protein Expression of FK / R2an Replicon by HCV Specific siRNAs
[0057]The Huh-7 cell line and its replicon cell line that stably supports HCV full-length replicon RNA of genotype 1b (Huh-7FK / R2AN) were obtained from Dr. S. K. Jang (PanBioNet, Korea). The FK / R2AN replicon is a modification from the HCV full-length replicon RNA, FK / RN (Korean Patent Publication No. 2004-32341), with the insertion of the signal sequence of foot-and-mouth disease virus (FMDV) 2A protease, which locates between Rluc and neo coding regions. Cell lines carrying HCV replicons were grown in DMEM containing 10% FBS (Gibco, USA) and 600 mg / ml G418 (Calbiochem, USA) and used in all experiments.
[0058]Transfection of DNAs of the siRNA expression plasmids or PCR products, or synthetic siRNA oligonucleotides was performed in 12-well plate using Lipofectamine 2000 reagent (Invitrogen, USA) in accordance with the manufacturer's instructions. Briefly, 1.5×105 Huh-7 FK / R2AN cells were plated, and the followi...
example 3
Reduction of Viral Transcripts by Vector-Mediated HCV siRNAs
[0067]Total RNA was extracted from FK / R2AN cells transfected with negative control or HCV replicon-specific siRNA expression vectors, at day 2 posttransfection, using Trizol LS reagent (Invitrogen, USA) according to the manufacturer's instruction. The isolated total RNA was digested with RNase-free DNase (Promega, USA). Finally, absolute amount of RNA was quantified by measuring UV-absorbance at 260 nm / 280 nm using UV-spectrophotometer.
[0068]In vitro antiviral activity was assessed by means of a quantitative real-time RT-PCR (Sequence Detection System 5700; Applied Biosystems, USA). The real-time RT-PCR was performed with 500 ng of total RNAs isolated from the transfected cells in a reaction volume of 50 μl using the TaqMan One-Step RT-PCR Master Mix Reagents (Applied Biosystems, USA). Standard RNA was prepared by in vitro transcription of 5′ UTR RNA by T7 RNA polymerase. The primer and probe sequences, specific for HCV 5′U...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com