Process for producing methionine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
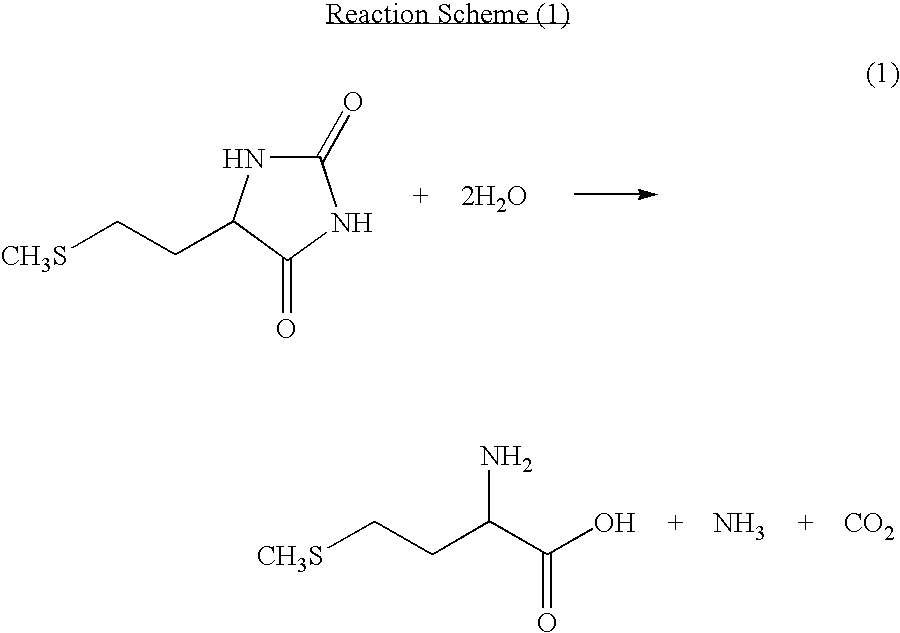
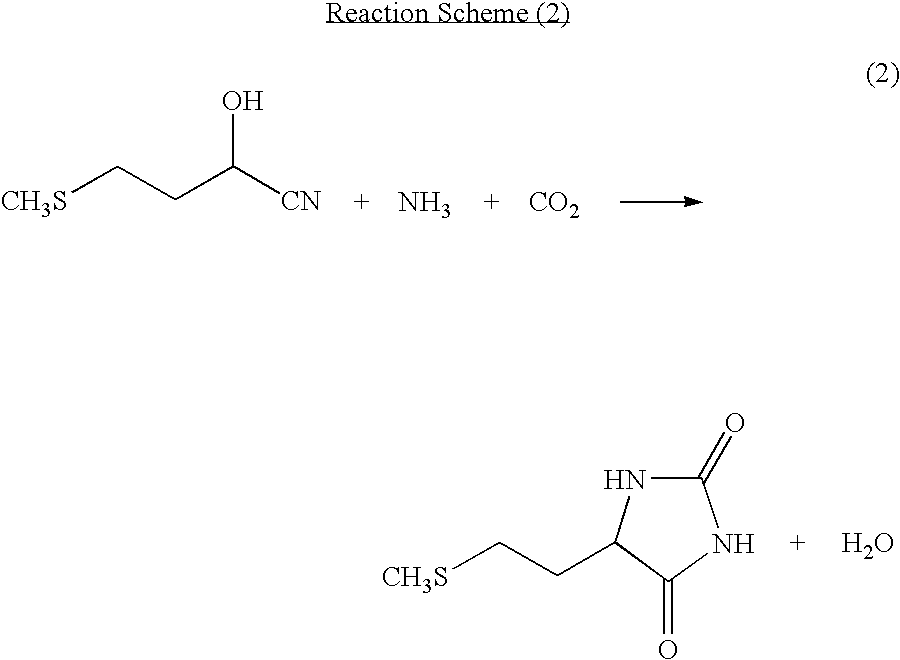
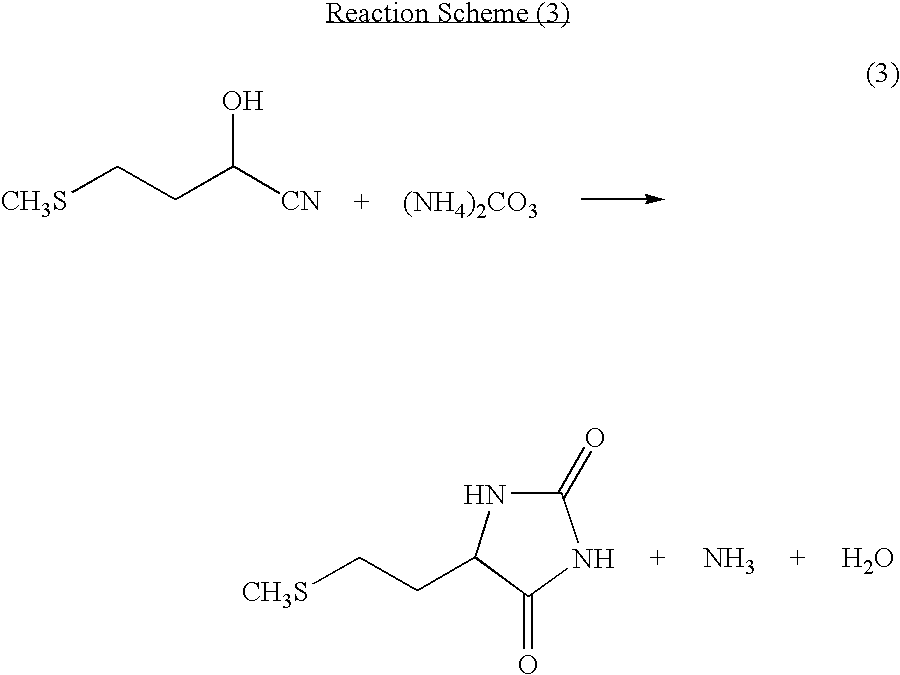
[0055]Into a non-stirred continuous first reaction tank, 5.4 parts per hour of a mixture of a solution containing 5-[2-(methylthio)ethyl]imidazolidine-2,4-dione and a potassium hydroxide aqueous solution was continuously charged. While carbon dioxide and ammonia produced as by-products were continuously distilled off together with water, the reaction solution was continuously charged into a second non-stirred continuous reaction tank which was different from the first reaction tank. At this point, the temperature of the reaction tanks was 177° C. and the gauge pressure was 0.88 MPa. The residence time in the first reactor was 30 minutes, and the residence time in the second reaction tank was 6 minutes, totaling 36 minutes. After reaction, the hydrolyzed solution thus obtained contained 10.4% of potassium, 13.1% of methionine, and 1.29% of methionine dipeptide, and the proportion of methionine dipeptide to methionine was 9.9%.
[0056]Into a reactor, 1 part of the hydrolyzed solution wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com