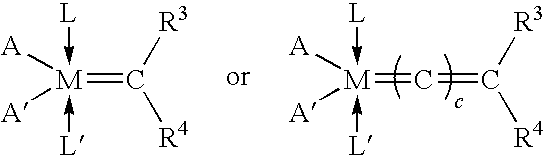
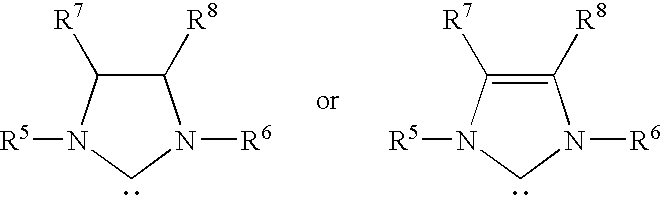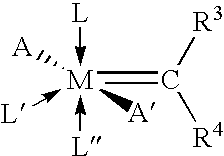Polyester Compositions Containing Metathesis Polymers with Reduced Recycle Color
a polymer and composition technology, applied in the field of aromatic polyester compositions, can solve the problems of compositions not desirable for recycling, limited packaging life of resins, and difficulty in recycling, and achieve the effect of reducing color formation, reducing leaching or migration of rubber polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Low Molecular Weight, Telechelic Acetate-Terminated Polypentenamer
[0083]A batch mixture comprised of 1.51 kg nitrogen-purged cyclopentene and 0.087 kg of 1,4-diacetoxy-2-butene were charged into a 1-gallon volume reactor, heated to 25° C., and stirred. A solution of 0.4 g of Grubbs' 2nd generation metathesis catalyst [Aldrich] in 100 mL dry, degassed toluene (0.45 mmol, 50,000:1 olefin:Ru) was charged into the vessel. Within 25 minutes, polymerization of the monomer began to occur, and a reaction temperature increase to 78° C. was observed. The resulting cement was then stirred at a constant 40° C. temperature for an additional 1 hour. At this time, a solution of 5 mL ethyl vinyl ether in 50 mL toluene was added to the polymer solution and stirred at 40° C. in order to deactivate any residual metathesis catalyst. The resulting polymer had the following characteristics: Mn=6.3 kg / mol; Mw / Mn=1.8; trans olefin content=78%; vinyl olefin content=0%; >95% acetate terminated e...
example 2
Synthesis of Telechelic, Low Molecular Weight Cyclooctene-1,5-Cyclooctadiene Copolymer
[0086]In a 1-gallon volume stainless steel reactor, a batch mixture was charged consisting of 1185 mL (996 g, 9.0 mol) of degassed cyclooctene, 560 mL (497 g, 4.6 mol) degassed 1,5-cyclooctadiene, and 60 mL (65.5 g, 0.38 mol) degassed cis-1,4-diacetoxy-2-butene. The mixture was stirred and heated to 50° C. A solution of 0.42 g (0.50 mmol) Grubbs' 2nd generation ruthenium metathesis catalyst in 10 mL dry, degassed toluene was prepared under inert atmosphere and added to the monomer mixture. Withing 1 minute, an increase in reaction temperature began to occur, with the peak temperature reaching 114° C. within 15 minutes. The reaction was stirred for 2 hours, after which a solution of 10 mL ethyl vinyl ether in hexanes was added to deactivate the metathesis catalyst. After stirring for 30 minutes, the polymer was dropped into bottles for storage and analyzed. The product polymer was an amber colored ...
example 3
Depolymerization of High Molecular Weight Polyoctenamer to Low Molecular Weight, Telechelic Cyclooctene-1,5-Cyclooctadiene Copolymer
[0087]In a 1-gallon volume stainless steel reactor, 830 g of polyoctenamer (Degussa Vestenamer 8012) pellets (Mn=65 kg / mol; Mw / Mn=1.8; 100% octenyl unit compositioni), 470 mL (417 g, 3.85 mol)degassed 1,5-cyclooctadiene, 40 mL (43.4 g, 0.25 mol) of degassed cis-1,4-diacetoxy-2-butene, and 700 mL of degassed hexanes were charged. The heterogeneous mixture was stirred and heated to 57° C. in order to melt the solid Vestenamer polymer. A solution of 0.22 g (0.26 mmol) Grubbs' 2nd generation ruthenium catalyst in 10 mL dry, degassed toluene was prepared under an inert atmosphere and added to the contents of the reactor. The reaction was stirred for 2 hours at a constant 57° C. temperature. After this period, a solution of 5 mL ethyl vinyl ether in 100 mL hexanes was added to deactivate the metathesis catalyst. After stirring for 30 minutes, the polymer was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melt temperature | aaaaa | aaaaa |
| melt temperature | aaaaa | aaaaa |
| melt temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com