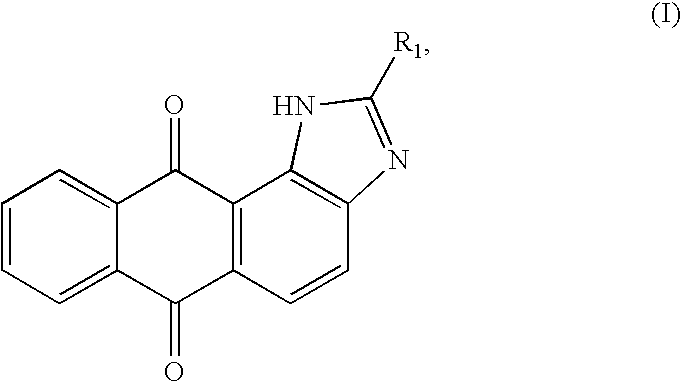
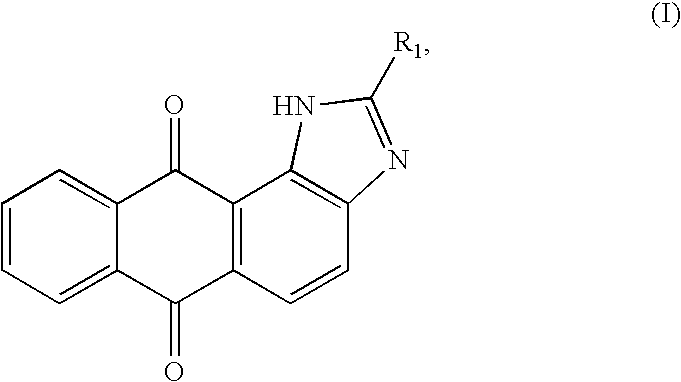
Heteroannelated anthraquinone derivatives and the synthesis method thereof
a technology of heterocyclic compound and anthraquinone, which is applied in the field of heterocyclic compound active ingredients, drug compositions, etc., can solve the problems of poor production rate, and achieve the effect of facilitating the study and application of cancer cells and inhibiting the proliferation activity of cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1 (
2-Methyl-1(3)H-anthra[1,2-d]imidazole-6,11-dione, No. 2)
[0030]1,2-Diaminoanthraquinone (1.19 g, 5 mmol) is dissolved in 30 mL of N,N-dimethylformamide, and chloroacetyl chloride (0.5 mL, 6 mmol) is added thereinto. After ten hours of mixing and reacting by a reverse flow, the mixture is transferred into 200 mL of icy water. After filtering, the precipitate is collected and washed by hot alcohol, so as to obtain the black compound No. 2.
[0031]The compound No. 2 has the following characterstics: MW 262.0724 (C16H9N2O2); Rf: 0.79 (ethyl acetate: dichloromethane=1:4); IR (KBr) cm−1: 1667 (CO); EI-MS m / z: 262 (M+, 100%); 1H-NMR (300 MHz, DMSO-d6) d (ppm): 2.72 (3H, s,—CH3), 7.75-7.82 (2H, m, Ar—H7,10), 7.93 (1H, d, J=8.4 Hz, Ar—H5), 8.13 (1H, d, J=8.4 Hz, Ar—H4), 8.19-8.23 (1H, m, Ar—H8,9), 11.01 (1H, br, —NH); and 13C-NMR (75 MHz, DMSO-d6) d (ppm): 23.89, 120.23, 121.22, 125.29, 126.19, 126.75, 127.19, 128.17, 128.87, 132.98, 134.18, 134.42, 148.22, 158.09, 182.43 (CO), 185.13 (CO).
embodiment 2 (
2-Chloroacetyl-1(3)H-anthra[1,2-d]imidazole-6,11-dione, No. 3)
[0032]
[0033]Except controlling the reacting temperature in 50-60° C., all steps are identical with the steps for manufacturing the compound No. 2, and the yellowish brown compound No. 3 can be obtained.
[0034]The compound No. 3 has the following characterstics: MW 296.0353 (C16H9N2O2Cl); Rf: 0.5 (ethyl acetate: dichloromethane=1:4); IR (KBr) cm−1: 3359(NH), 1660 (CO); HRMS (ESI-TOF) m / z: calcd for C16H10N2O2Cl+ [M+H]+: 297.0425, found: 297.0426; 1H-NMR (300 MHz, CDCl3) d (ppm): 4.92 (2H, s, —CH2Cl), 7.80-7.83 (2H, m, Ar—H7,10), 8.08 (1H, d, J=8.4 Hz, Ar—H5), 8.24(1H, d, J=8.4 Hz, Ar—H4), d8.26-8.35(2H, m, Ar—H8,9), d11.21(1H, br, —NH); and 13C-NMR (75 MHz, DMSO) d (ppm): 37.80, 119.35, 121.27, 125.95, 126.83, 127.40, 129.06, 132.35, 133.47, 133.64, 134.88, 135.10, 148.89, 156.93, 183.04 (CO), 183.83 (CO).
embodiment 3 (
2-Ethyl-1(3)H-anthra[1,2-d]imidazole-6,11-dione, No. 4)
[0035]
[0036]1,2-Diaminoanthraquinone (1.19 g, 5 mmol) was dissolved in dimethylformamide (30 mL), and propionaldehyde (0.29 g, 5 mmol) is added thereinto. Concentrated sulfuric acid (0.1 mL) is added thereinto for catalyzation. After mixing and reacting at room temperature for one hour, the reacted mixture is transferred into 200 mL of icy water and is extracted by using dichloromethane. The extract is dried, and crystallized by using alcholo, so as to obtain the brown compound No. 4.
[0037]The compound No. 4 has the following characterstics: MW 276.0899 (C17H12N2O2); Rf: 0.75 (ethyl acetate: dichloromethane=1:4); IR (KBr) cm−1: 1669 (CO); HRMS (ESI-TOF) m / z: calcd for C17H13N2O2+ [M+H]+: 277.0971, found: 277.0975 calcd for C17H12N2O2Na+ [M+Na]+: 299.0971, found: 299.0794; 1H-NMR (300 MHz, CDCl3) d (ppm): 1.51 (3H, t, J=7.5 Hz, —CH3), 3.05 (2H, q, J=7.5 Hz, —CH2—), 7.73-7.81 (2H, m, Ar—H7,10), 7.99 (1H, d, J=8.4 Hz, Ar—H5), d8.16...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com