Commercial production of polysaccharide degrading enzymes in plants and methods of using same
a technology of polysaccharide degrading enzyme and plant, which is applied in the field of commercial production of heterologous proteins in plants, can solve the problems of crippling the ability of the economy to function, affecting the standard of living of the population, and consuming over 100 billion gallons of gasoline per year in the transportation sector alon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Plasmids
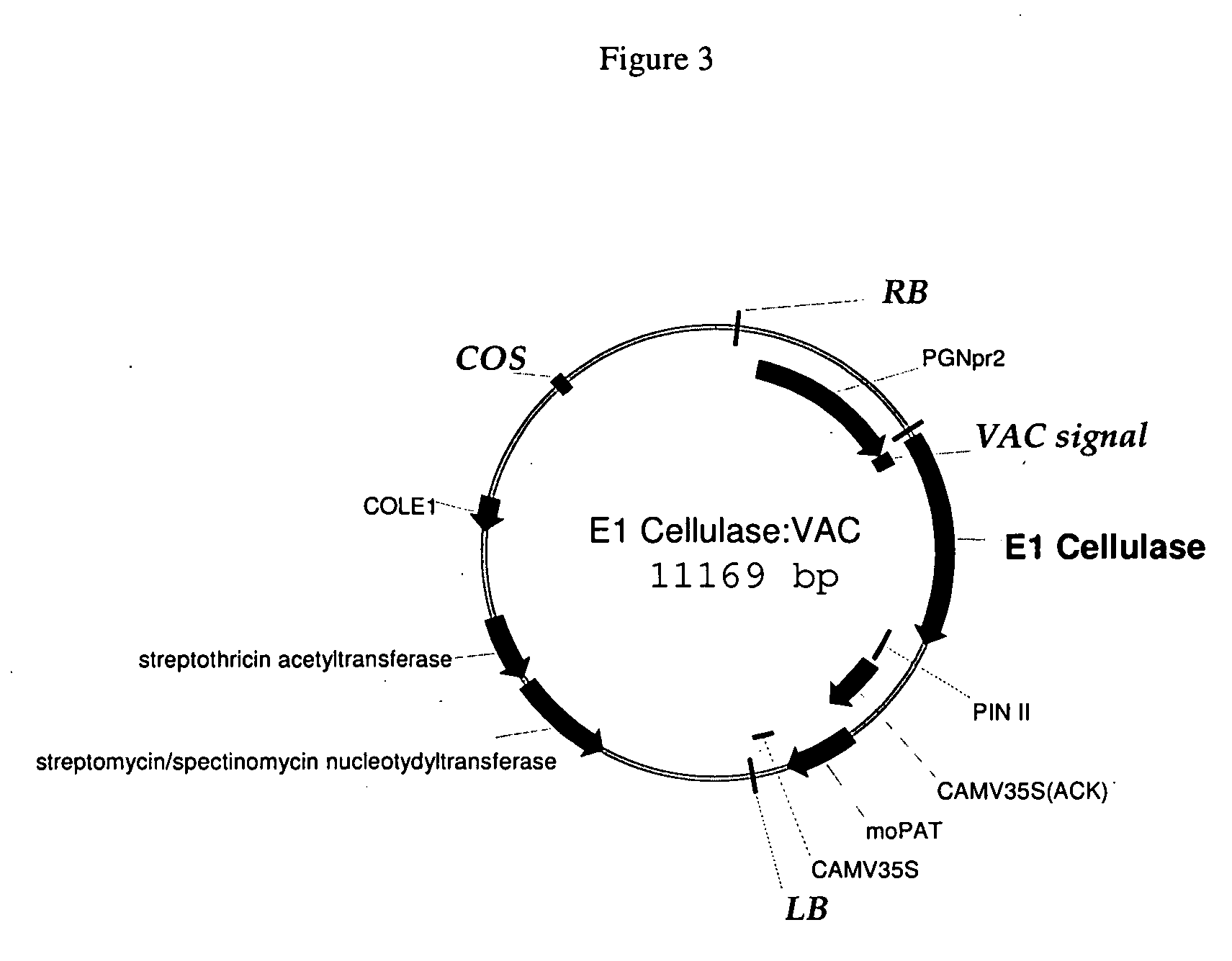
[0134]FIG. 1 shows the E1 vector, having the E1 cellulase sequence (FIG. 2, SEQ ID NO: 1), the seed-preferred promoter PGNpr2 (supra), the KDEL (SEQ ID NO: 12) endoplasmic reticulum retention sequence shown in FIG. 4A (SEQ ID NO: 2); the barley alpha-amylase signal sequence, (BAASS), which was optimized and is shown in FIG. 4B (SEQ ID NO: 3), and a pin II terminator, supra. The 35S promoter, supra, drives the selectable marker, the maize optimized PAT gene. The gene confers resistance to bialaphos. See, Gordon-Kamm et al, The Plant Cell 2:603 (1990); Uchimiya et al, Bio / Technology 11:835 (1993), and Anzai et al, Mol. Gen. Gen. 219:492 (1989). The E1 cellulase gene from Acidothermus cellulolyticus was received from NREL. For expression in maize, the first 40 amino acids were optimized to maize preferred codons. The BAASS and KDEL (SEQ ID NO: 12) sequences were added to the gene by PCR using the NREL clone as template. The PCR product moved to a PCR-ready clonin...
example 2
Transformation of Maize
[0138]Fresh immature zygotic embryos were harvested from Hi-II maize kernels at 1-2 mm in length. The general methods of Agrobacterium transformation were used as described by Japan Tobacco, at Ishida et al. 1996. “High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens” Nature Biotechnology 14:745-750 with the modifications described supra. Fresh embryos were treated with 0.5 ml log phase Agrobacterium strains EHA101. Bacteria were grown overnight in a rich medium with kanamycin and spectinomycin to an optical density of 0.5 at 600 nm, pelleted, then re-inoculated in a fresh 10 ml culture. The bacteria were allowed to grow into log phase and were harvested at no more dense than OD600=0.5. The bacterial culture is resuspended in a co-culture medium.
[0139]For stable transformations, embryos were transferred to a bialaphos selective agent on embryogenic callus medium and transferred thereafter every two weeks to allow growth o...
example 3
Enzyme Analysis
[0140]Six single seed from each plant (up to 10 plants per event) were assayed separately. Each seed was pulverized in an automatic seed pounder and extracted in a high-speed shaker in 1 ml of 50 mM sodium acetate, pH 5. Cell debris was pelleted and the supernatant recovered for analysis of 1) total soluble protein using the Bradford assay (Bradford, M. 1976. Anal. Biochem. 72:248) and 2) the concentration of the target protein using the assay described below.
[0141]The E1 enzyme concentration was determined through the following activity assay. The assay is performed in a microtiter plate format. An appropriate amount of extract from transgenic seed containing 1 ug of TSP is transferred to a well of a 96-well microtiter plate. The total sample volume is brought to 0.1 ml with the addition of extraction / reaction buffer. The reaction is started with the addition of 0.025 mL of 5 mM 4-methylumbelliferyl-m-D-cellobioside (MUC). The reaction is incubated at 50° C. for 30-4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dry weight | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
| dry weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com