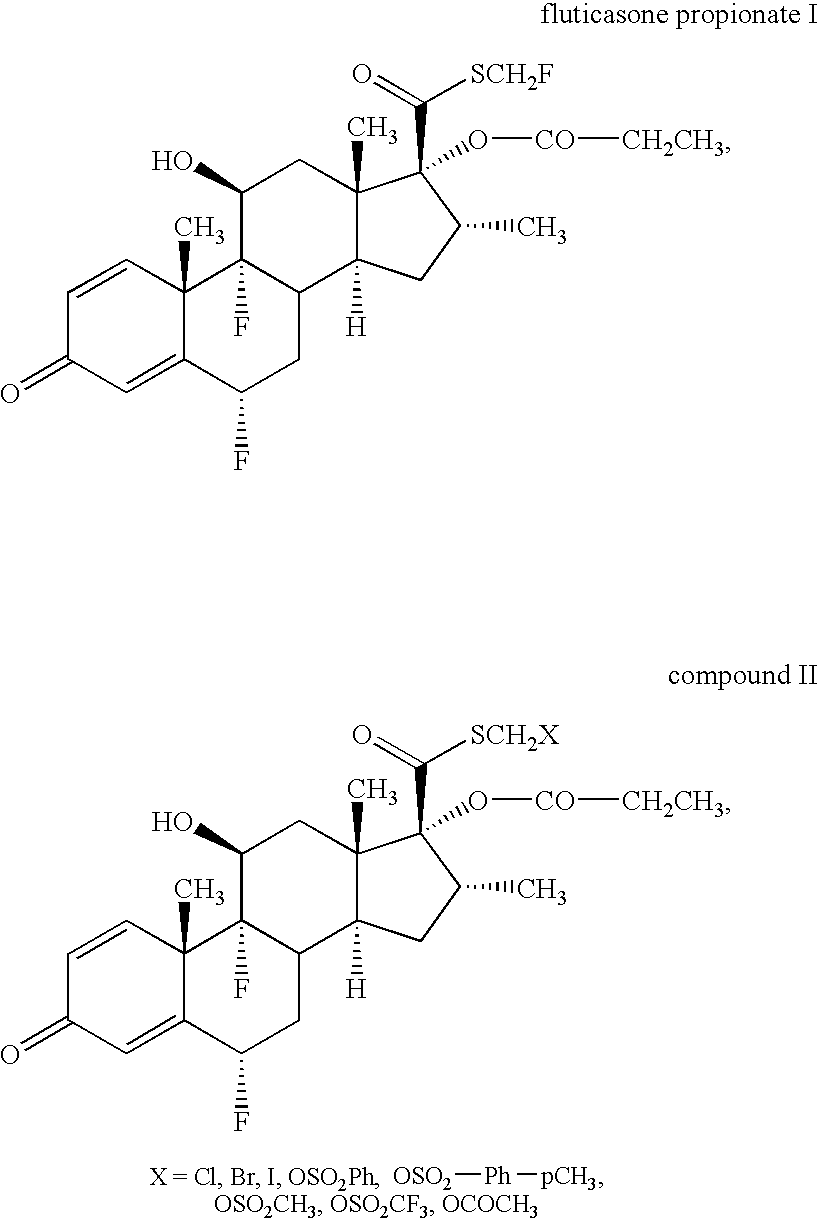
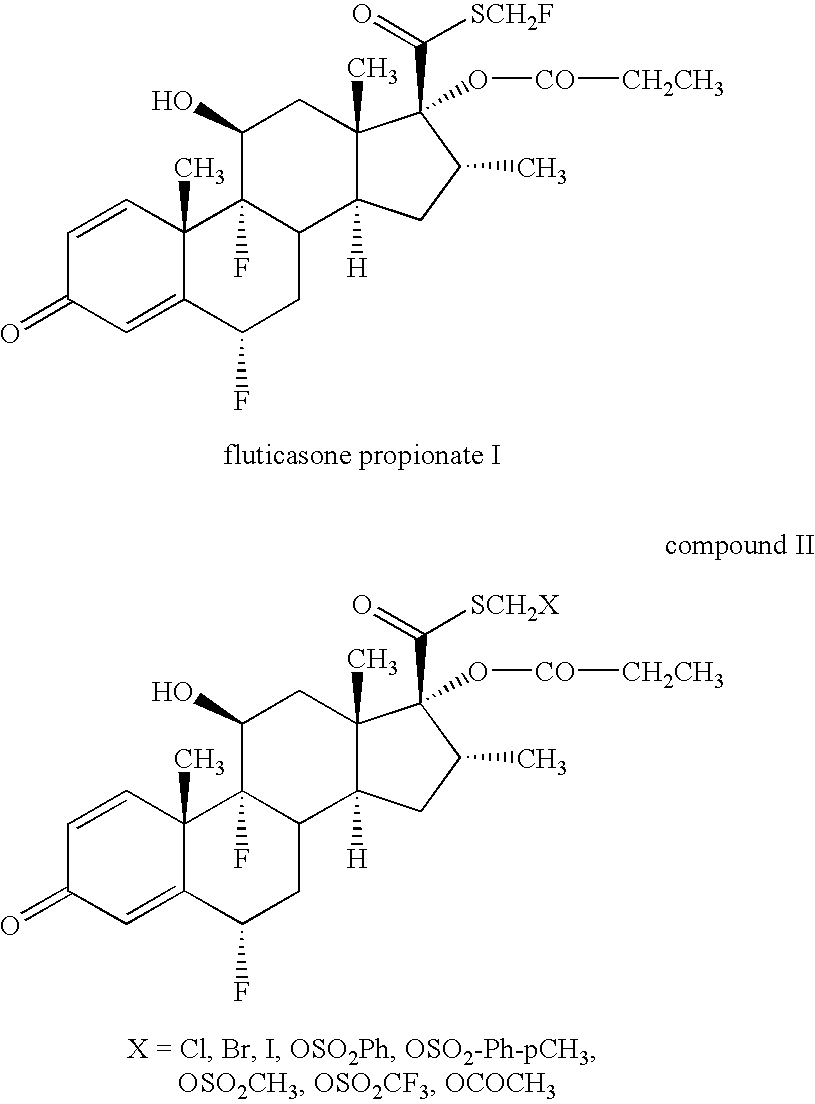
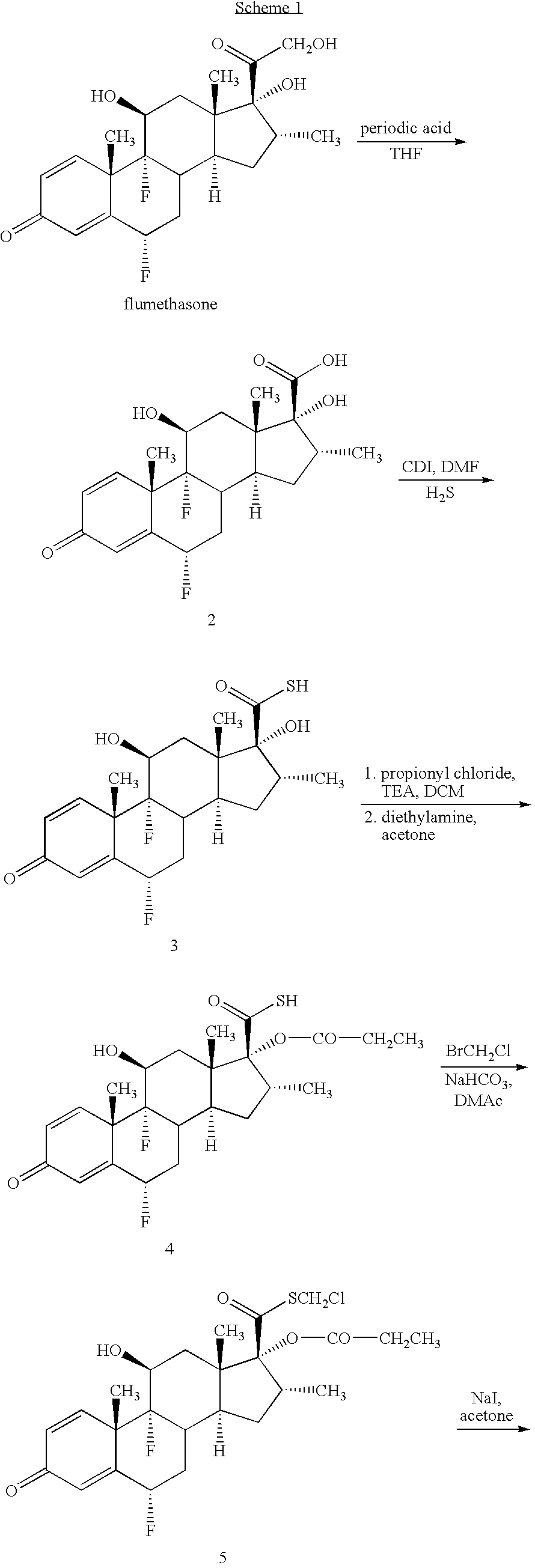
Novel process and intermediates
a technology of thioates and thioates, applied in the field of process for the preparation of steroidal 17carboxylic thioates, can solve the problems of affecting the quality of steroids, and affecting the effect of endocrine system disorder, so as to achieve convenient commercial scale production and convenient and efficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example a
[0129]A solution of mixed fluorides was obtained by refluxing silver fluoride (10.0 eq) and calcium fluoride (10.0 eq) in acetonitrile at 90-95° C. for 4 hours, followed by filtration. Compound 6 was charged at −10 to −15° C. into the solution obtained and the reaction mixture was stirred for 36 hours at −10 to −15° C. Then further silver fluoride (2.0 eq) was added and the reaction mixture was stirred for a further 12 hours at −10 to −15° C. Crude fluticasone propionate I was isolated by aqueous extraction work up using ethyl acetate, Na2CO3, 2M HCl and water. Distillation of the ethyl acetate, followed by swapping with diisopropyl ether gave colourless fluticasone propionate I. By following this procedure, crude fluticasone propionate I was obtained with more than 98% HPLC purity consistently (Table 4).
TABLE 4HPLC purity%(area %) of crudeYieldfluticasoneExp no.Reaction conditions(w / w)propionate IExp-1acetonitrile filtrate of AgF (10.0 eq)6098.15and CaF2 (10.0 eq), S-iodo compound6...
example b
[0130]Fluticasone propionate I obtained from the above example was further crystallised using methanol (55 vol) at 60-65° C. The clear solution was treated with activated carbon and then filtered. By chilling the resulting solution to 0 to −5° C. for maximum isolation, pure product was isolated. This was dried under reduced pressure at 50-55° C. This gave fluticasone propionate I with more than 99% HPLC purity and conforming to the EP 5.0 and USP 29 specifications. The non-polar dimeric impurities (impurities H and I in EP 5.0; impurities D and E in USP 29) were significantly below (˜0.10%) the specified pharmacopoeial limit (0.2% in EP 5.0, 0.3% in USP 29), see Table 5.
TABLE 5Crystallisation%HPLC purity (area %) of purifiedsolventsYieldfluticasone propionate I and RRTExp no.and conditions(w / w)of impurities wrt EP 5.0 / USP 29Exp-1Methanol (55 vol),71Related substances as per EP 5.0reflux at 60-65° C.,fluticasone propionate I: 99.52activated carbon,Impurity C: 0.10chilling to 0 toImpu...
example 1
Preparation of Iodomethyl Ester 6 from Chloromethyl Ester 5
[0131]Sodium iodide (4.0 eq) was charged to acetone (20 vol) under stirring. Butylated hydroxytoluene (BHT) (1.0 eq) and hydroquinone (1.0 eq) were added to the stirred suspension of sodium iodide at 25-30° C. The reaction mixture was stirred for 30 minutes. Chloromethyl ester 5 (1.0 eq) was added to this stirred suspension and the reaction mixture was refluxed for 24 hours at 60-65° C. After completion of the reaction, the product was isolated by distillation of acetone and precipitation by adding 5% w / v solution of NaHCO3. The crude iodomethyl ester 6 was filtered, washed with water (3×10 vol) and dried under reduced pressure (˜100 mm of Hg) at 55-60° C. for 4 hours. Yield: 75-85% w / w. HPLC purity: 96-97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com