All-solid-state cell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
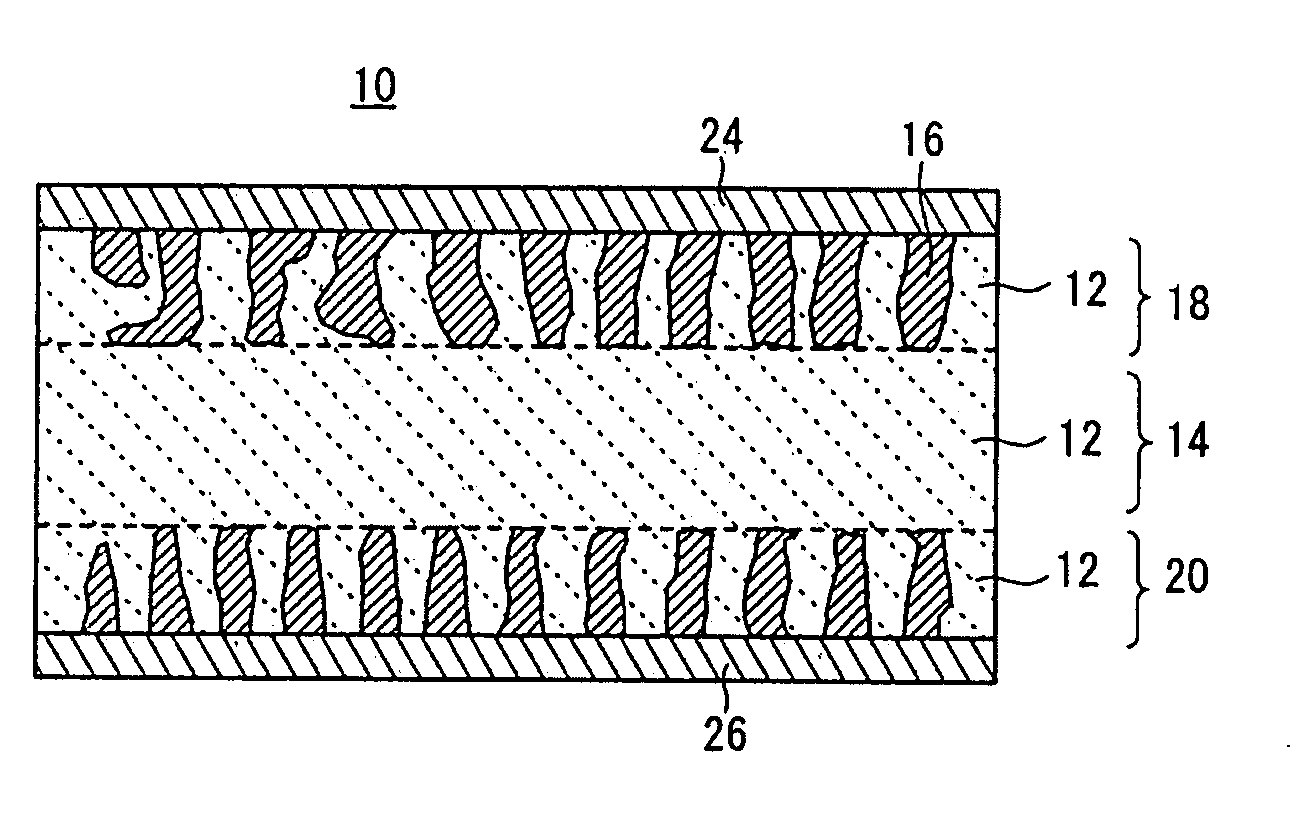
[0107]A binder was dissolved in an organic solvent, and an appropriate amount of the resultant solution was added to the LAGP glass powder and the LVP crystal powder. The mixture was kneaded in a mortar to prepare an electrode paste for screen printing. The obtained electrode paste was printed into an electrode pattern having a diameter of 12 mm on each surface of a fired solid electrolyte body (a base) having a diameter of 13 mm and a thickness of 1 mm. The printed electrode patterns were dried to form positive and negative electrodes.
[0108]The electrodes were bonded to the surfaces of the solid electrolyte base by firing at 600° C. for 2 hours using a firing furnace under an Ar atmosphere. Then, a sputtered gold (Au) film having a thickness of approximately 50 nm was formed as a collector on each surface of the resultant fired body.
[0109]After the firing, the positive electrode had a thickness of approximately 20 μm and an active material content of approximately 2 mg. The charge-...
example 2
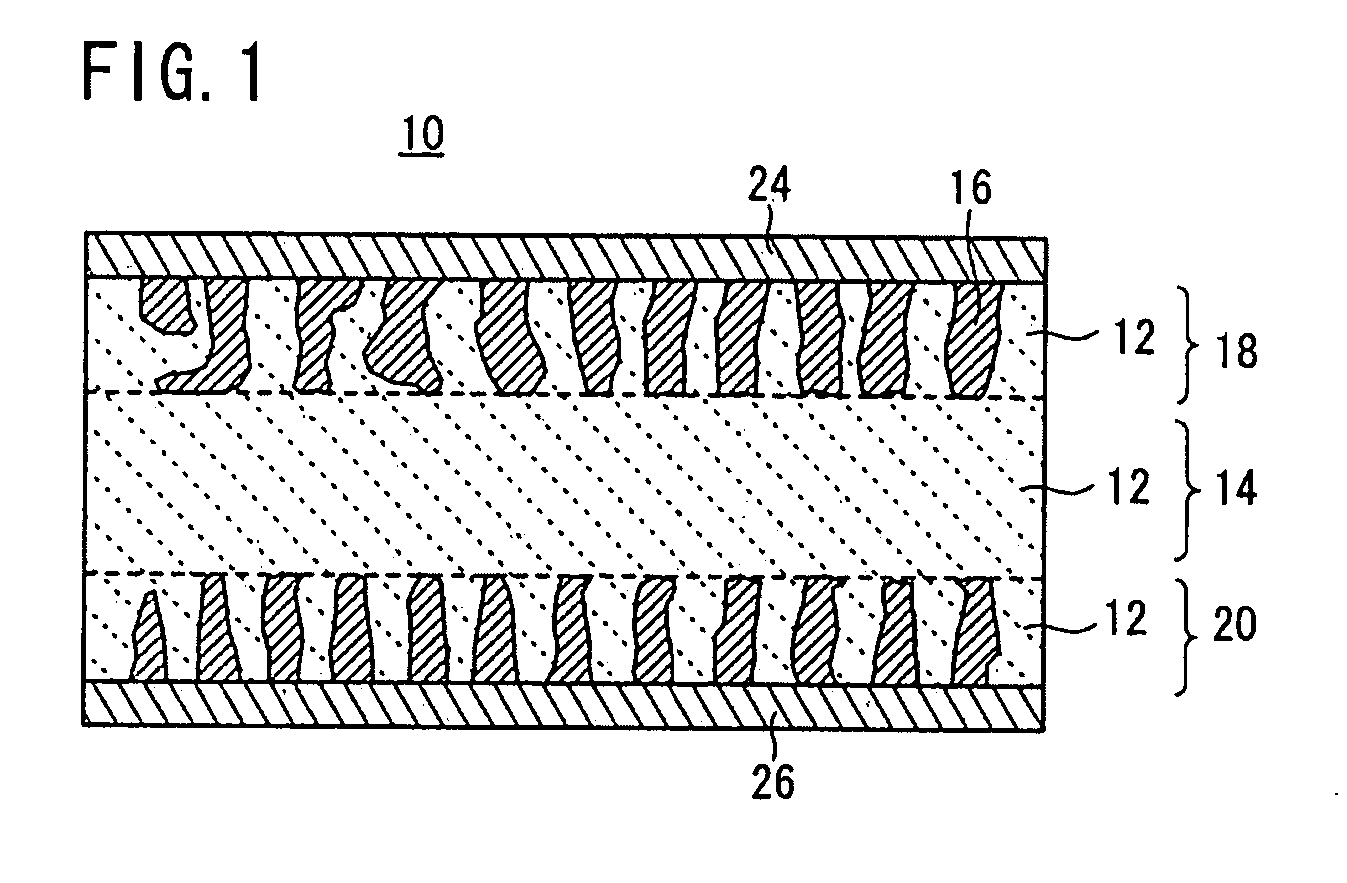
[0110]A binder was dissolved in an organic solvent, and an appropriate amount of the resultant solution was added to the LAGP glass powder and the LVP crystal powder. The mixture was kneaded in a mortar to prepare an electrode paste for screen printing. The obtained electrode paste was printed into an electrode pattern and dried on each surface of a fired solid electrolyte body (a base) in the above-mentioned manner, to form positive and negative electrodes.
[0111]The electrodes were bonded to the surfaces of the solid electrolyte base by firing at 600° C. for 40 hours using a firing furnace under an Ar atmosphere. Then, a sputtered Au film was formed on each surface of the resultant fired body.
[0112]After the firing, the positive electrode had a thickness of approximately 20 μm and an active material content of approximately 2 mg as above.
example 3
[0124]An all-solid-state cell of Example 3 was produced, and the charge / discharge property and the alternating-current impedance property were measured.
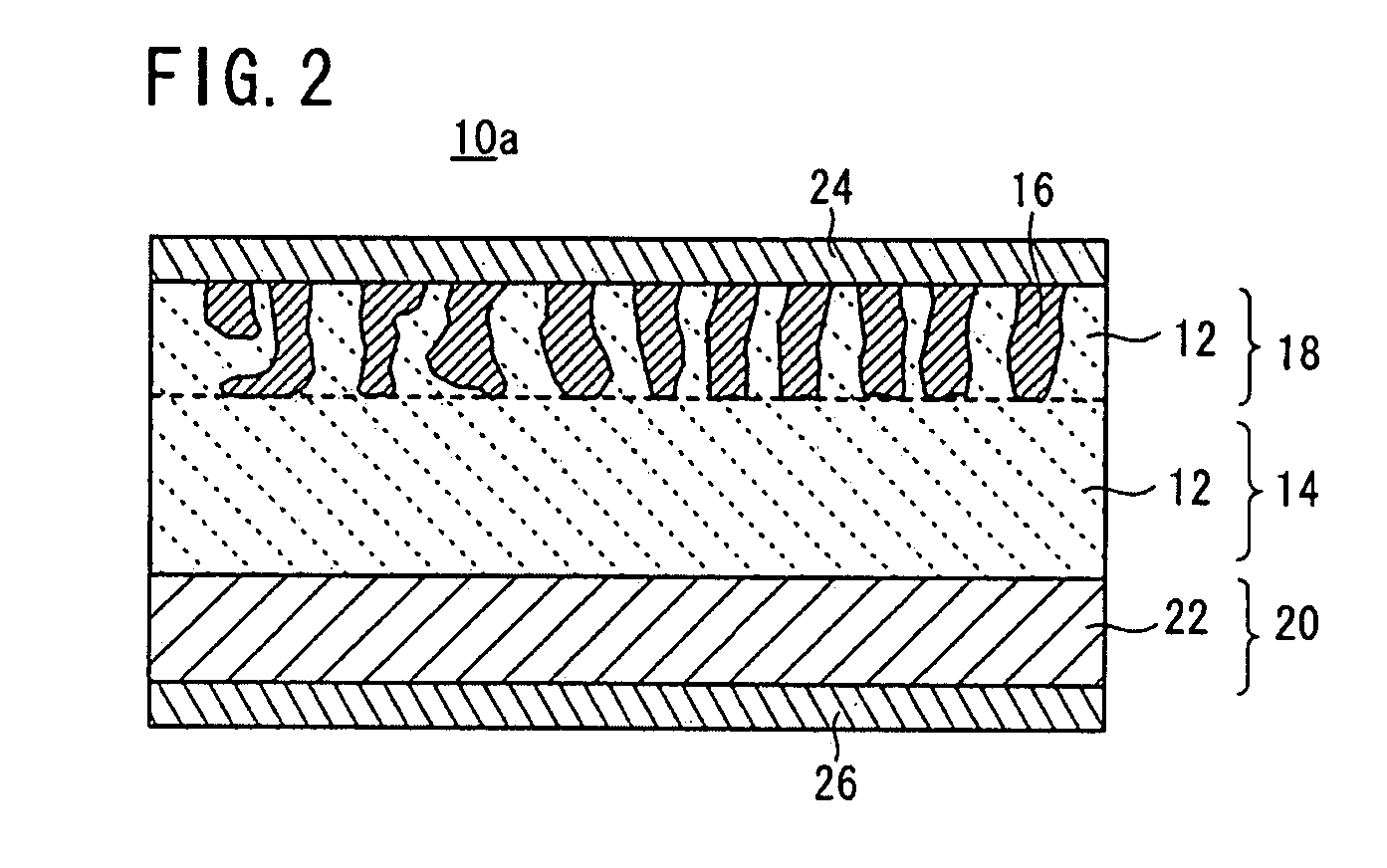
[0125]In the same manner as Example 1, a binder was dissolved in an organic solvent, and an appropriate amount of the resultant solution was added to the LAGP glass powder and the LVP crystal powder. The mixture was kneaded in a mortar to prepare an electrode paste for screen printing. The obtained electrode paste was printed into an electrode pattern having a diameter of 12 mm on each surface of a fired solid electrolyte body (a base) having a diameter of 13 mm and a thickness of 1 mm. The printed electrode patterns were dried to form positive and negative electrodes.
[0126]The electrodes were bonded to the surfaces of the solid electrolyte base by firing while applying a load of 500 kg / cm2 in the thickness direction at 600° C. for 40 hours using a hot-press furnace under an Ar atmosphere. Then, a sputtered gold (Au) film having a th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com