Novel hemopoietin receptor protein, nr12
a technology of hemopoietin and receptor protein, applied in the field of new hemopoietin receptor protein, can solve the problems of difficult strict selection, unsatisfactory consensus sequence of yr motif, and difficult screening under normal hybridization experiment conditions, so as to increase durability and membrane permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
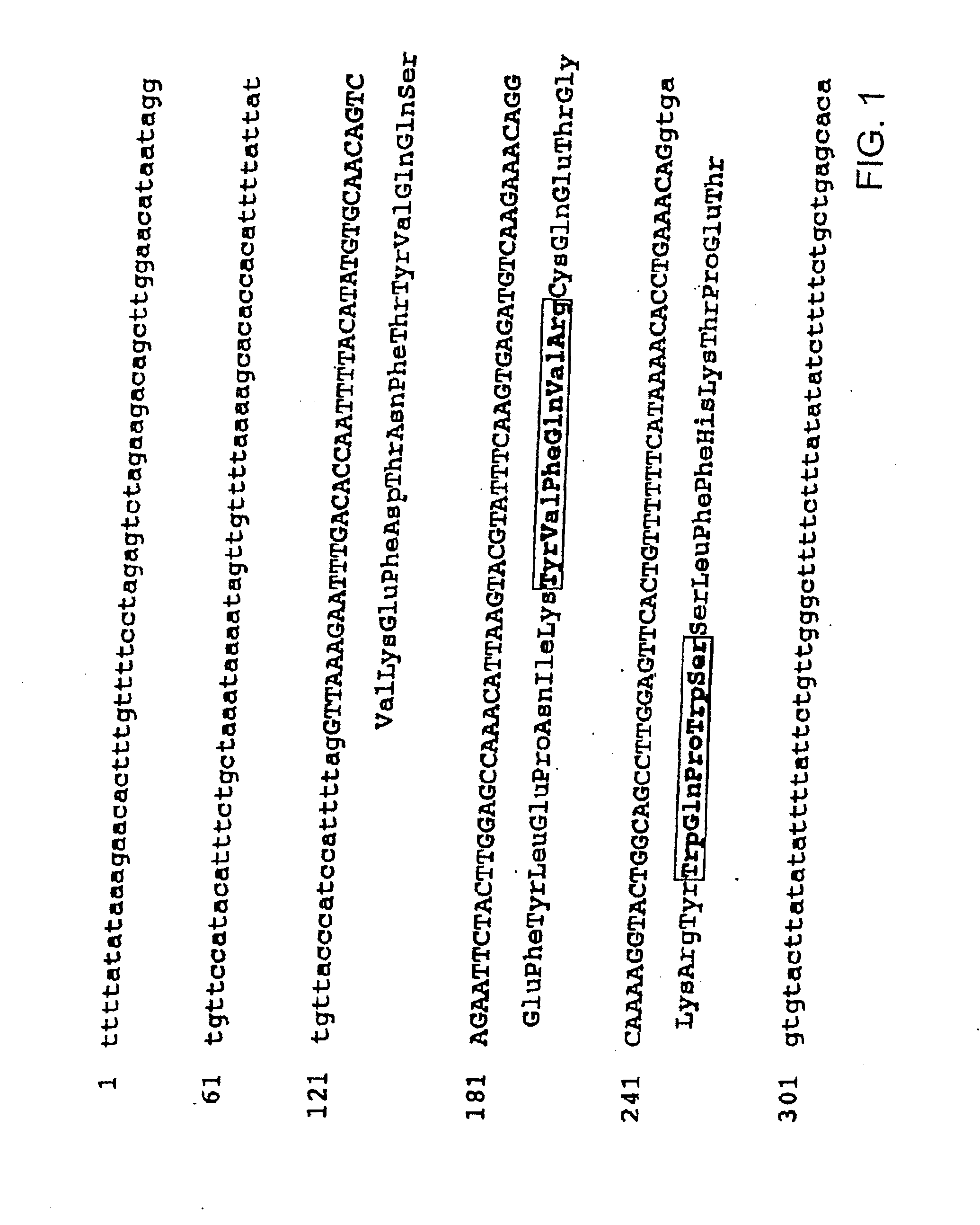
Isolation of NR12 Gene
(1) Primary Screening by TblastN Search
[0177]Although sequencing of human genome is promoted extensively by human genome projects of institutes, the proportion of completely finished sequences to the whole human genome has not reached even 10%. However, information provided by above projects until today is counted as a good means for searching target genes, determining nucleotide sequences, and mapping genes. The informational basis of the above sequences consists of large information provided by the assembly of bacterial artificial chromosome (BAC) and yeast artificial chromosome (YAC), which aims to form a complete database in the future. The present inventors identified a human gene encoding a part of a novel hemopoietin receptor protein from a BAC clone sequence in one of public databases, “High Throughput Genomic Sequence (htgs)” of GenBank.
[0178]As mentioned above, the present inventors found motif sequences conserved in the hemopoietin receptor family, n...
example 2
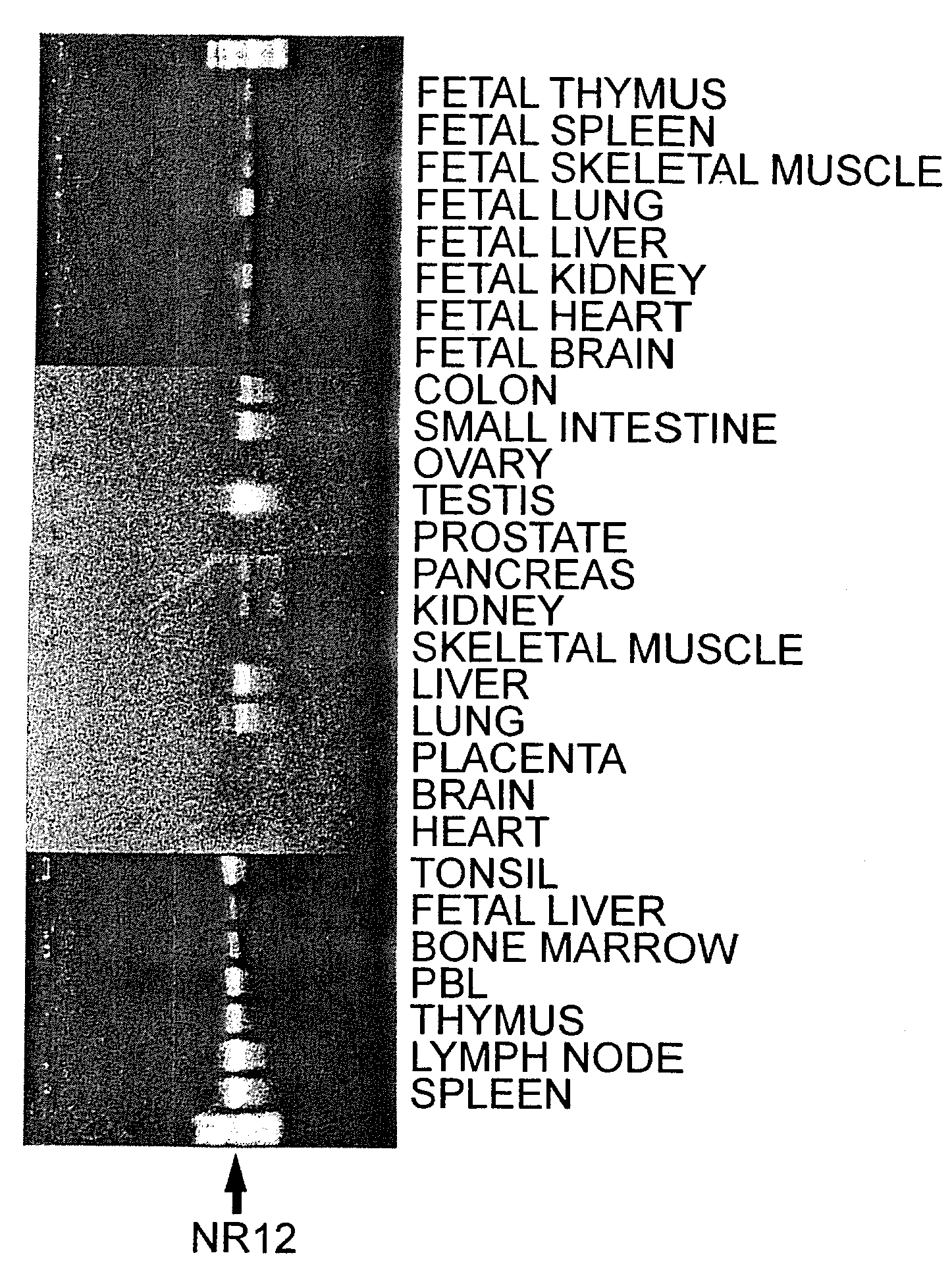
Tissue Distribution Determination and Expression Pattern Analysis of NR12 Gene by RT-PCR
[0200]mRNA was detected using the RT-PCR method to analyze the expression distribution and the expression pattern of NR12.1 gene in different human organs. NR12-PPD primer with the sequence below was synthesized as a sense primer (downstream orientation) for the RT-PCR analysis. NR12-A1 primer synthesized in Example 1 (3) was used as the antisense primer (upstream orientation). The NR12-PPD primer was synthesized and purified as in Example 1 (3). It was expected that the common N-terminal region in all splice variants, NR12.1, NR12.2 and NR12.3, are amplified and detected using these primer sets (NR12-PPD and NR12-A1).
hNR12-PPD;(SEQ ID NO:17)5′-CCG CCA GAT ATT CCT GAT GAA GTA ACC-3′
[0201]The templates used were Human Multiple Tissue cDNA (MTC) Panel I (Clontech #K1420-1), Human MTC Panel II (Clontech #K1421-1), Human Immune System MTC Panel (Clontech #K1426-1), and Human Fetal MTC Panel (Clontech...
example 3
Verification of the Specificity of RT-PCR Product by Southern Blotting
[0206]In order to verify the specificity of amplification, the RT-PCR amplified target gene product in Example 2 was subjected to Southern blotting using NR12 specific cDNA fragment as a probe. At the same time, the amount of RT-PCR product was quantitatively detected by the strength of labeled signal to assess relative gene expression levels among different human organs. The RT-PCR product was electrophoresed on an agarose gel, blotted onto a charged nylon membrane, Hybond N (+) (Amersham, cat #RPN303B), and was subjected to hybridization. The 5′-RACE PCR product cDNA fragment corresponding to the N-terminus of the NR12 obtained in Example 1 (4) was used as a probe specific to NR12. Probes were prepared using the Mega Prime Kit (Amersham, cat #RPN1607), and labeled with radioisotope, [α-32P] dCTP (Amersham, cat #AA0005). Hybridization was performed using Express Hyb Hybridization Solution (Clontech #8015-2), and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com