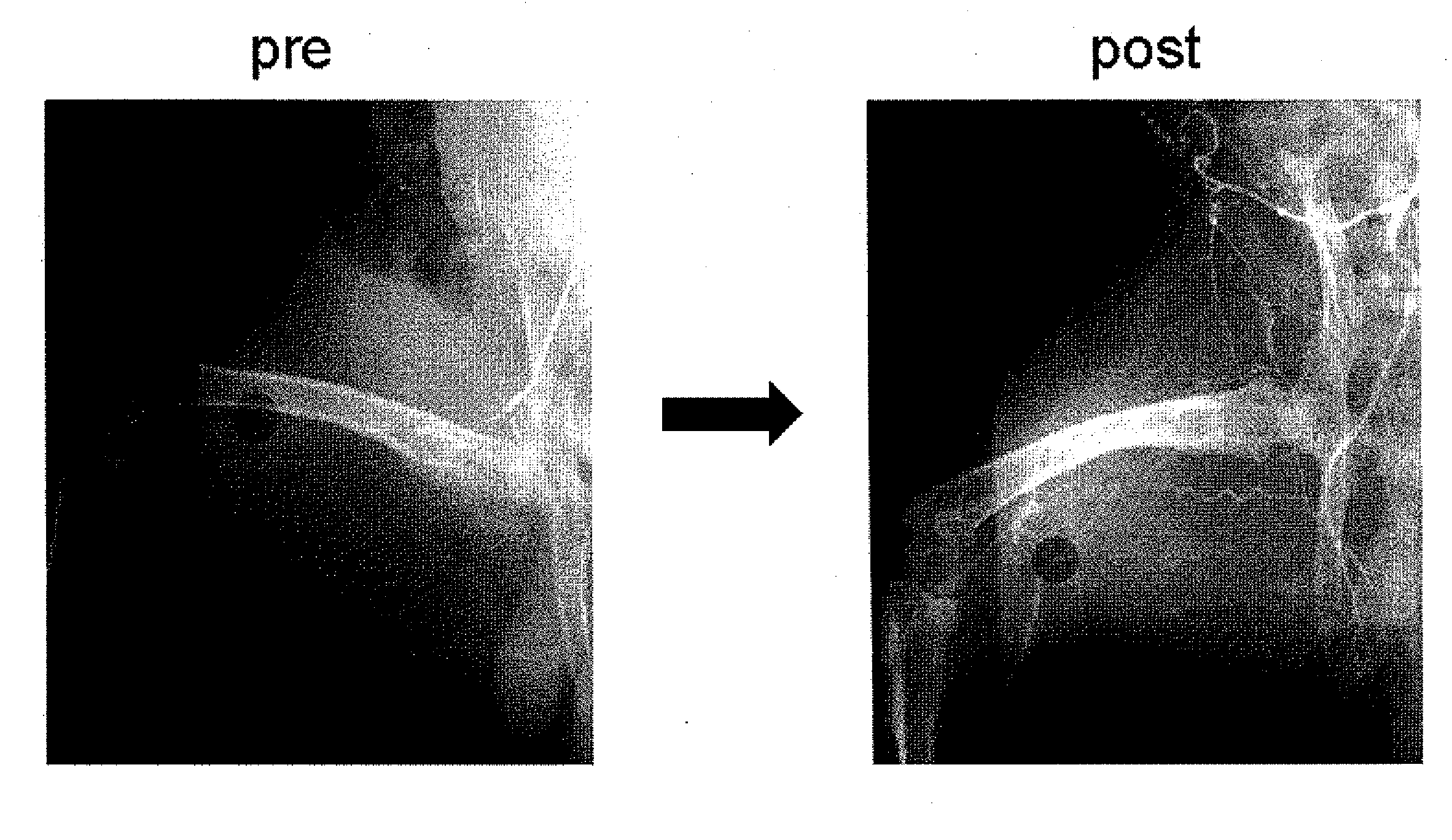
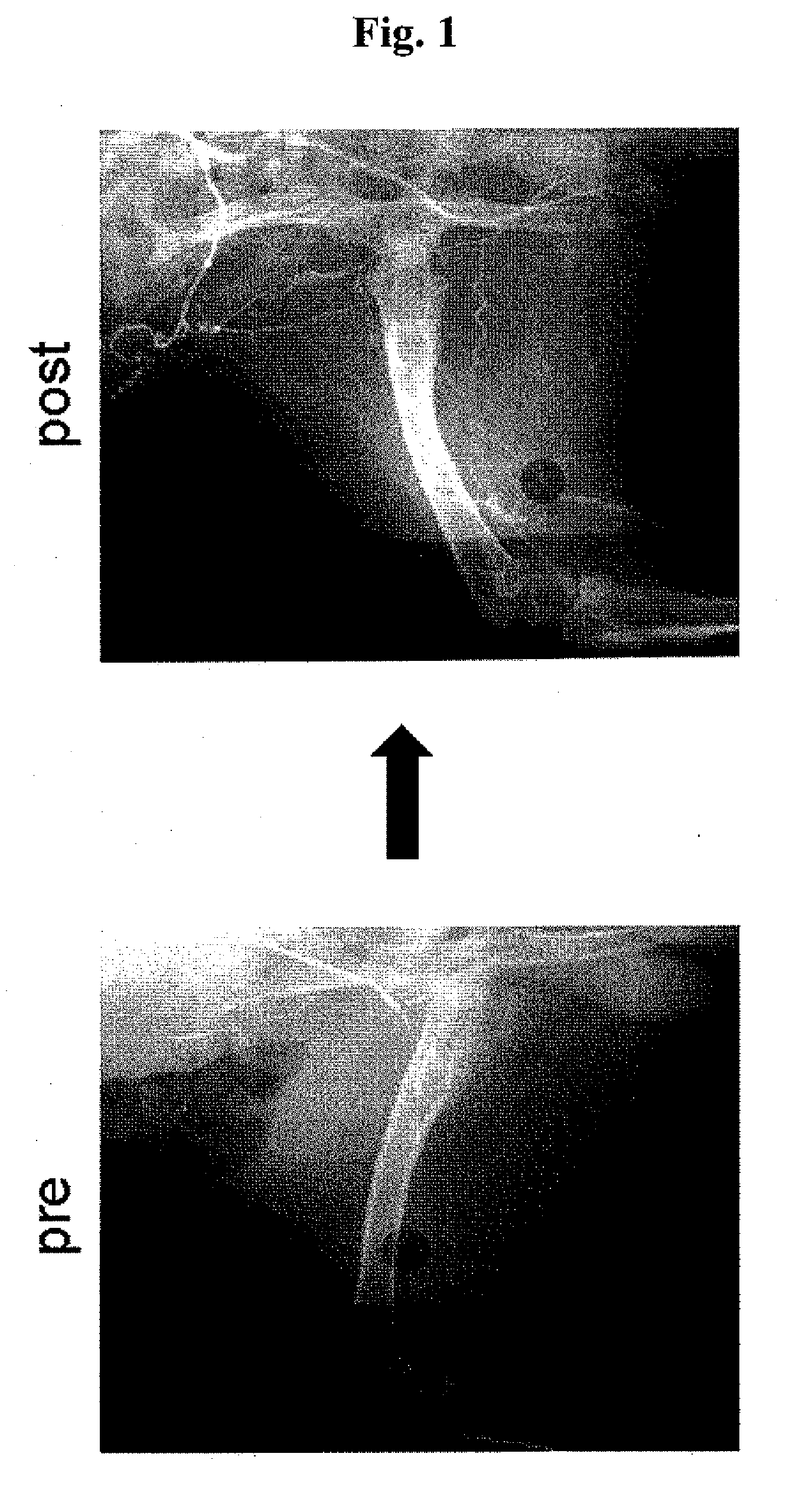
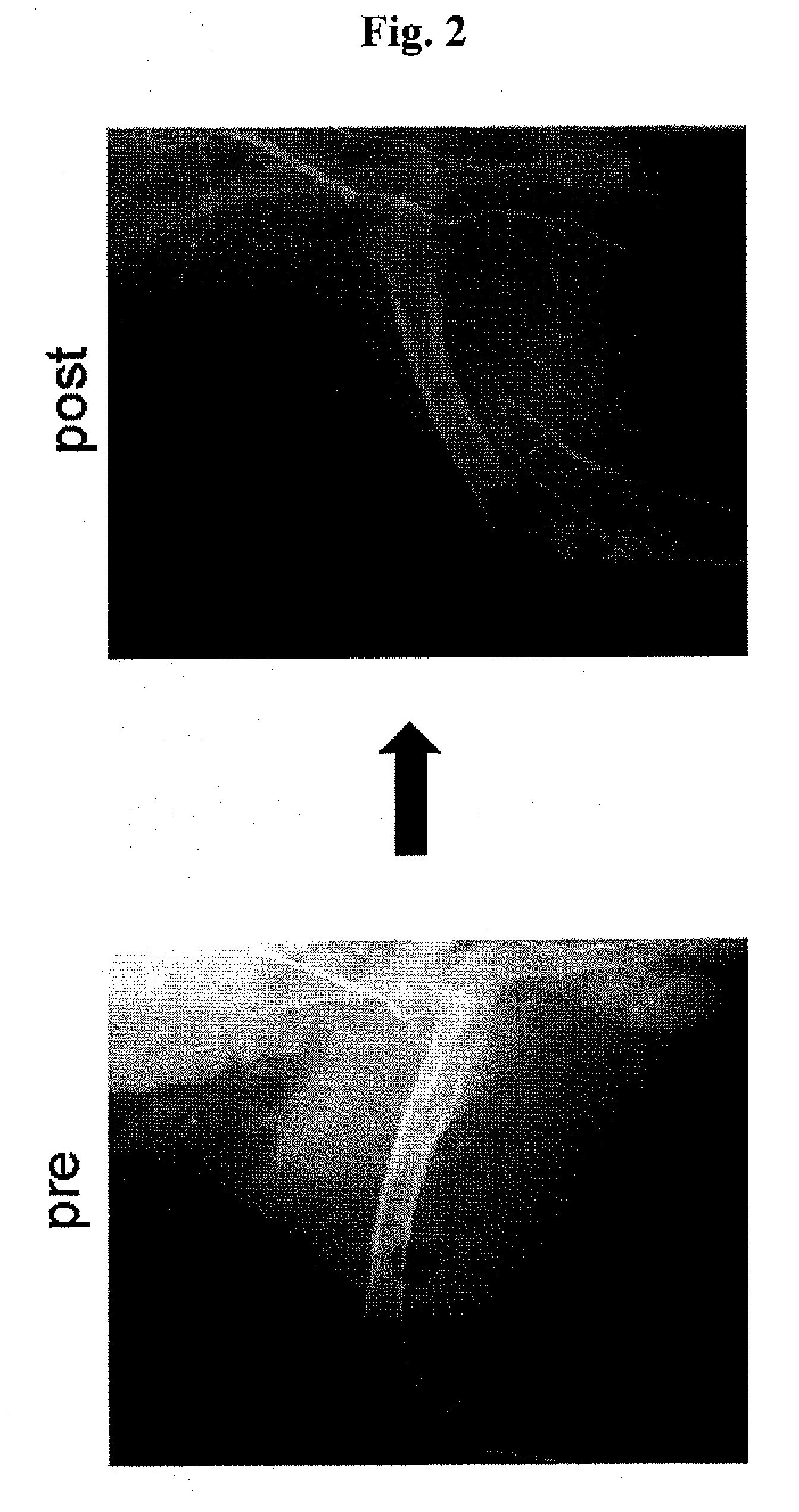
Ischemia therapeutic agent
a technology of ischemia and therapeutic agents, applied in the field of ischemia therapeutic agents, can solve the problems of refractory infections, delayed wound healing, intense psychological and physical suffering of patients, etc., and achieve the effects of promoting vascularization, preventing reperfusion injury, and inhibiting fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
A. Preparation of bFGF-Containing Gelatin Hydrogel
[0059]Gelatin hydrogel containing bFGF was prepared according to the method described in WO 94 / 27630. More specifically, a mixture of alkaline-treated gelatin aqueous solution having an isoelectric point of 4.9 (10 wt %, 20 ml) and olive oil (5 ml) was preheated at 40° C. and stirred for 1 hour, and after removing the natural gelatin by cooling the prepared emulsion with ice, acetone was added followed by stirring for 1 hour at 4° C. The resulting gelatin particles were washed three times with acetone (4° C.) and then recovered by centrifugal separation (5000 rpm, 4° C., 5 minutes).
[0060]The resulting non-crosslinked gelatin particles (20 mg) were suspended in an aqueous solution of Tween 80 (0.1%, 20 ml) containing glutaraldehyde (0.13 wt %) and then stirred for 24 hours at 4° C. to carry out the crosslinking reaction. After recovering by centrifugal separation (5000 rpm, 4° C., 5 minutes), the particles were stored for 1 hour at 37...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com