Sequential lixivation and precipitation of metals from refractory ores by utilising variable oxidation reduction potentials and a variable PH system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
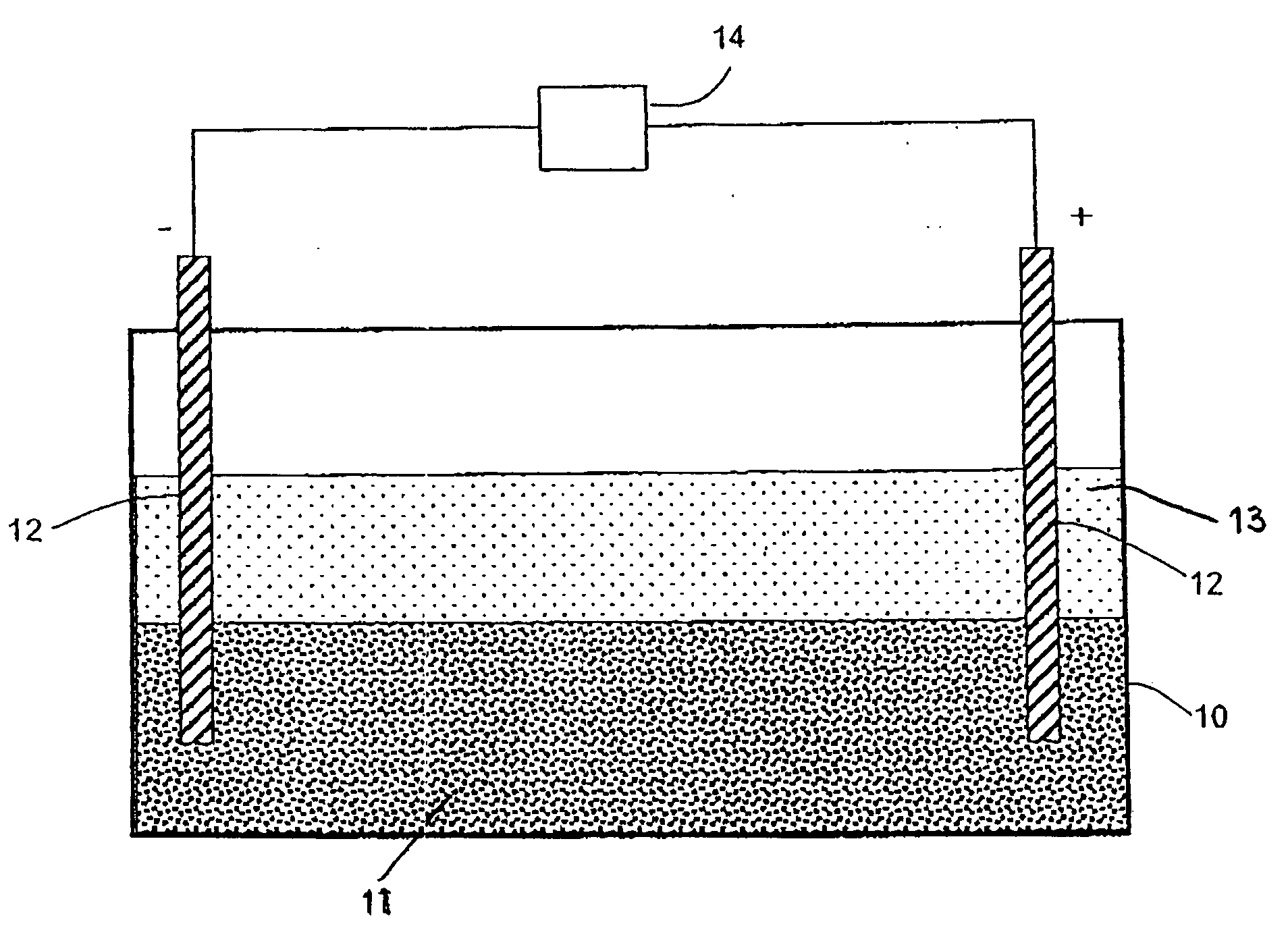
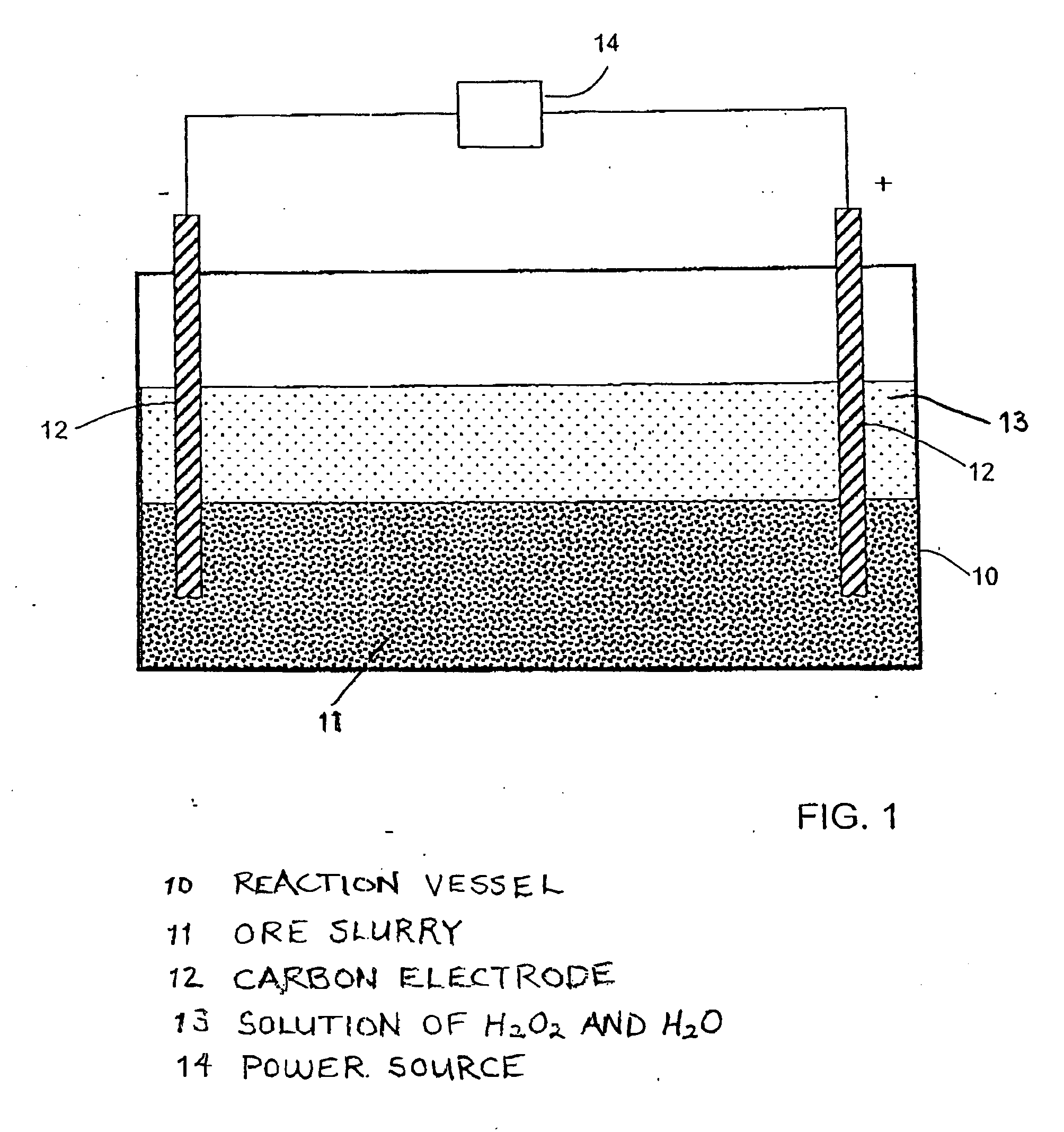
[0031]Tailings from Crystal Graphite Corporations mine near the city of Slocan, British Columbia were tested. One pound (454 grams or 15 assay tons) of tailings were placed in a plastic leach container. A solution comprising 3000 cc water (H2O), 150 cc hydrogen peroxide (H2O2), and 150 cc concentrated hydrochloric acid (HCl) was added to the tailings to form a slurry. Two graphite electrodes were placed spaced apart into the slurry in the container, and a twelve volt six ampere current was applied for a period of three hours. The leached solution was then filtered. The resulting acid solution was then neutralized with sodium hydroxide (NaOH) so that a dark brown precipitate formed, which was then filtered out and dried. After drying, 38 g of precipitate remained which was then fire assayed and cupelled. This produced a 3.2 mg metal bead which was found by Assayers Canada to contain $52 per ton in gold.
example 2
[0032]A 200 gram sample of siliceous rock with garnets in it was placed in a plastic reaction vessel containing an undiluted mixture of hydrochloric acid (HCl) and hydrogen peroxide (H2O2) and a six volt current was applied for six hours. At that time it was found that the garnets were untouched and the rock matrix was partially dissolved. After leaving the sample in the solution for another six hours, it was found that the remaining rock had dissolved along with some of the garnets. The solution was then diluted and the metals precipitated. On assay, it was found that the metal values were
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com