Material and Methods for Nerve Grafting
a nerve grafting and material technology, applied in the field of nerve grafting materials and methods, can solve the problems of pain, neuronal death, muscle atrophy and permanent functional deficit, and the inability to achieve full restoration of function after nerve transection repair, so as to improve the ability of regenerating axons, promote the repair of damaged nerve tissue, and increase the number of axons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Degradation of CSPG by Treatment of Acellular Nerve Segments with Chondroitinase
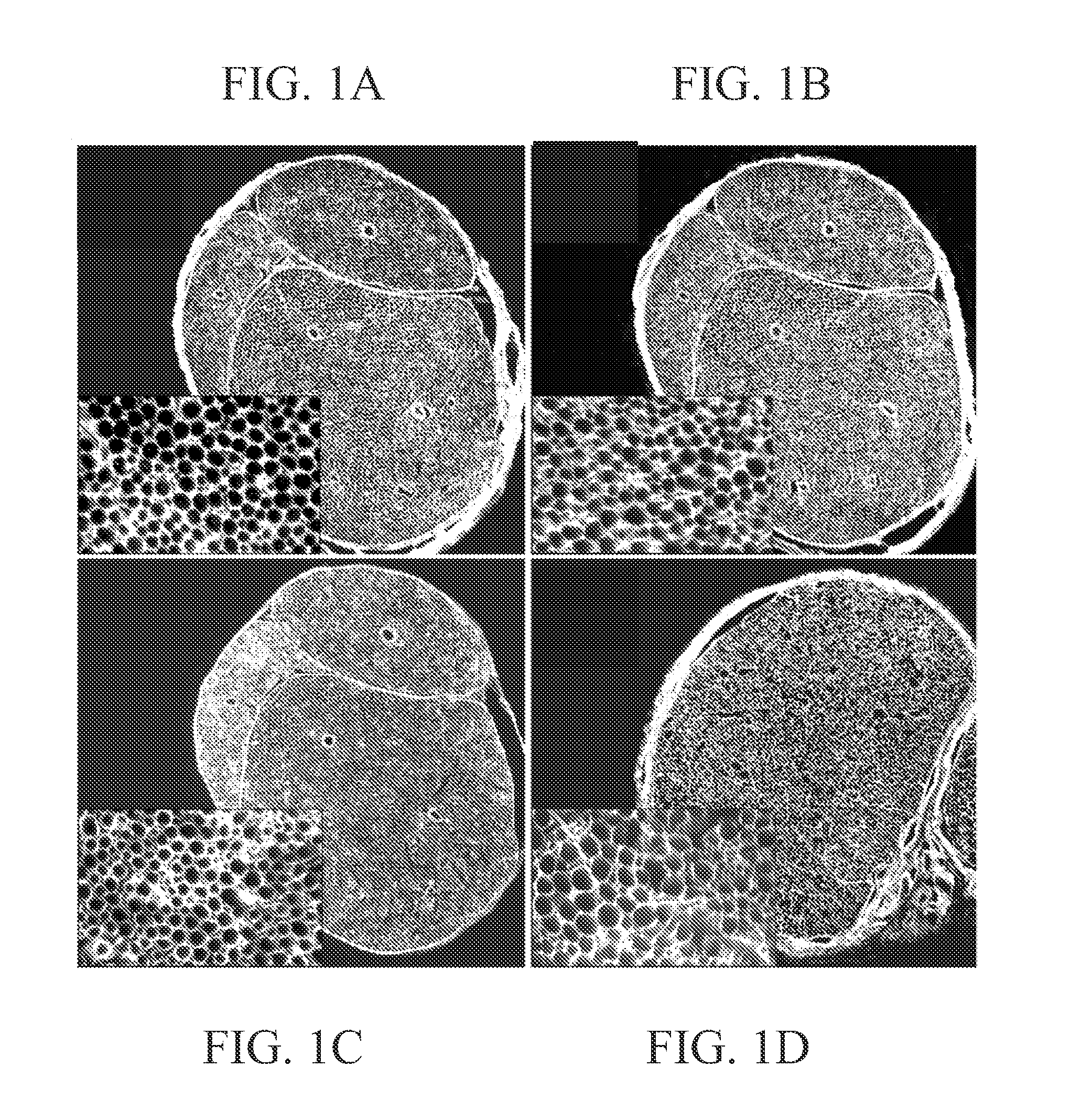
[0125]The purpose of this experiment was to determine if chondroitinase treatment effectively degraded CSPG throughout intact segments of acellular nerves. Segments of rat sciatic nerve (1.5 cm in length) were made acellular by repeated freeze-thaw cycles and then bathed en bloc in a chondroitinase ABC solution for 16 hours. CSPG degradation within the chondroitinase pretreated nerves was examined by immunolabeling with neoepitope antibody Ab1918. This antibody binds to an epitope created on the core protein after lysis of the chondroitin sulfate chains by chondroitinase ABC (Bertolotto et al. [1986] J. Neurol Sci, 73:233-244). Ab1918 immunostaining was intense throughout the entire pretreated nerve segment, as shown in FIG. 1A. Furthermore, the intensity of Ab1918 immunostaining was not increased by an additional post-treatment of the sections with chondroitinase, as shown in FIG. 1B. Ab1918 immunoreact...
example 2
Inactivation of Inhibitory CSPG by Treatment of Acellular Nerve Segments with Chondroitinase
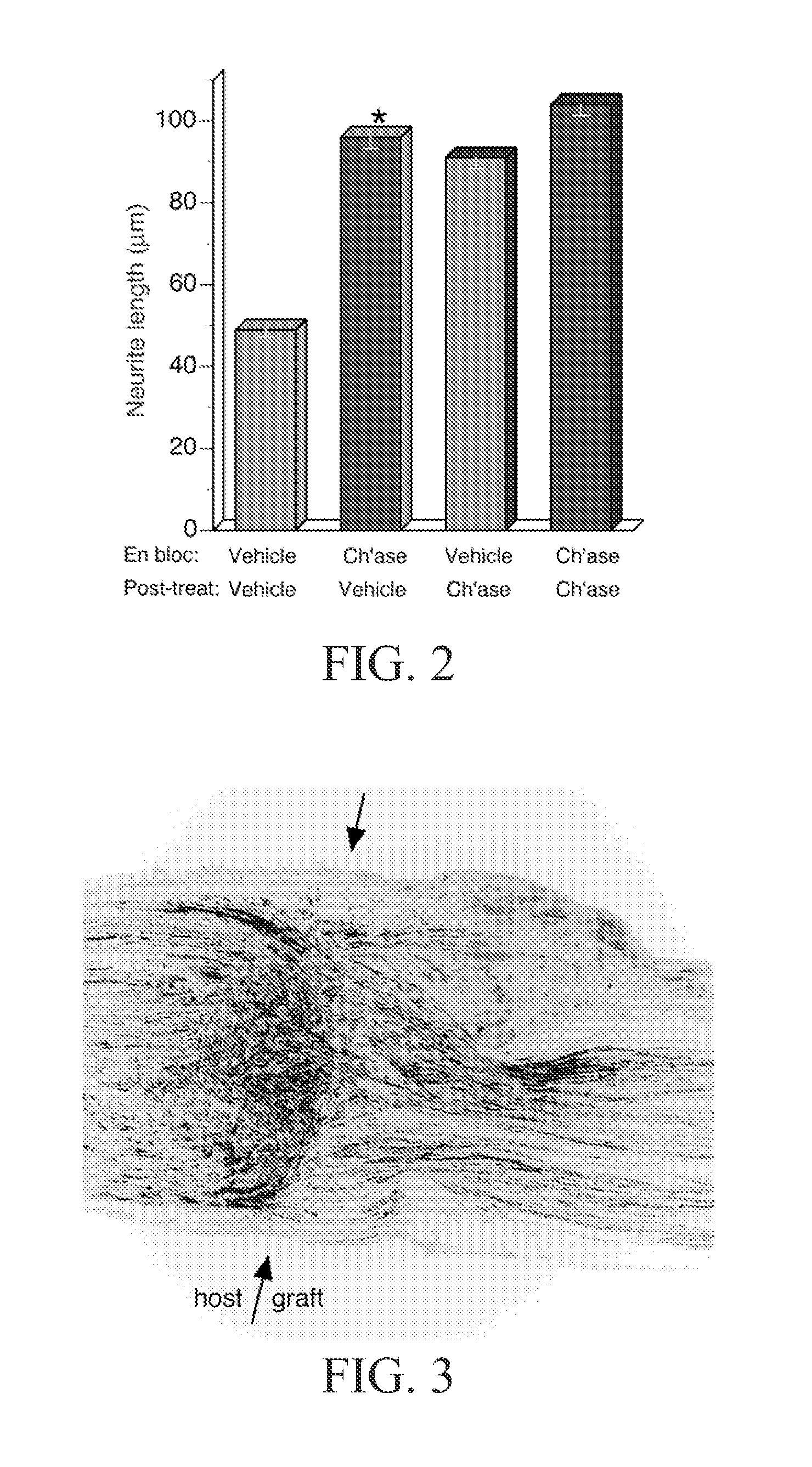
[0127]Inactivation of inhibitory CSPG in chondroitinase-treated acellular nerve was determined by cryoculture bioassay. Embryonic chick DRG neurons were seeded onto sections of prepared nerve segments and the neurite-promoting activity was assessed by scoring neurite growth. Results are shown in FIG. 2. On sections of acellular nerve pretreated en bloc with vehicle only the average neurite length was 49 μm. Neurite growth on acellular nerve pretreated en bloc with chondroitinase averaged 96 μm, representing a 95% increase compared to the control condition. To determine if the en bloc chondroitinase treatment was thorough, cryoculture assays were performed on nerve tissues treated with chondroitinase after sectioning (post-treatment). As expected, the neurite-promoting activity of acellular nerve treated en bloc with vehicle only was increased significantly (86%) by post-treatment with chondro...
example 3
Nerve Regeneration is Enhanced by Chondroitinase Treatment of Acellular Nerve Grafts
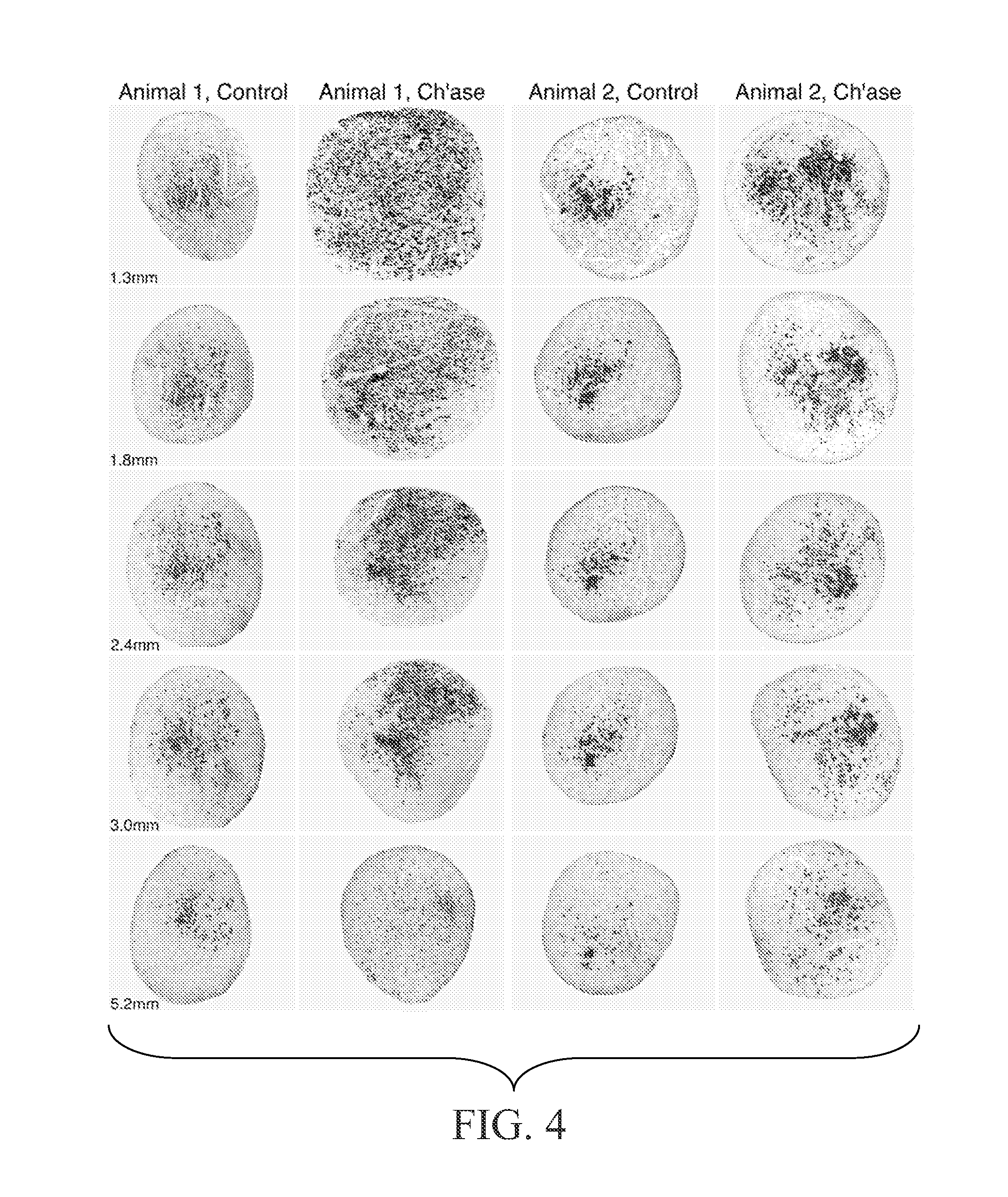
[0129]The following experiments tested the hypothesis that chondroitinase treatment improves nerve regeneration through acellular nerve syngrafts. As described in Example 2, acellular sciatic nerve segments were treated en bloc with vehicle or chondroitinase ABC. Ten-mm interpositional nerve grafts were joined to the host nerve by epineurial neurorrhaphy reinforced with fibrin glue. Each of nine host rats received bilateral grafts, one vehicle-treated and one chondroitinase-treated graft. Regeneration was initially examined after 8 days. First, the proximal and distal nerve-graft coaptations were examined in longitudinal section to assess the alignment of the surgical coaptation, as shown in FIG. 3. All of the grafts were in continuity and thus were included in the subsequent analysis. Scoring of regeneration was based on GAP-43-immunolabeling which intensely stained growing axons. Axon and Schwann c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com