Device and Methods for Suturing Tissue
a tissue and suture technology, applied in the field of tissue suture devices and methods, can solve the problems of increased incidence of complications, ischemia and/or thrombosis, and uncomfortable procedures for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
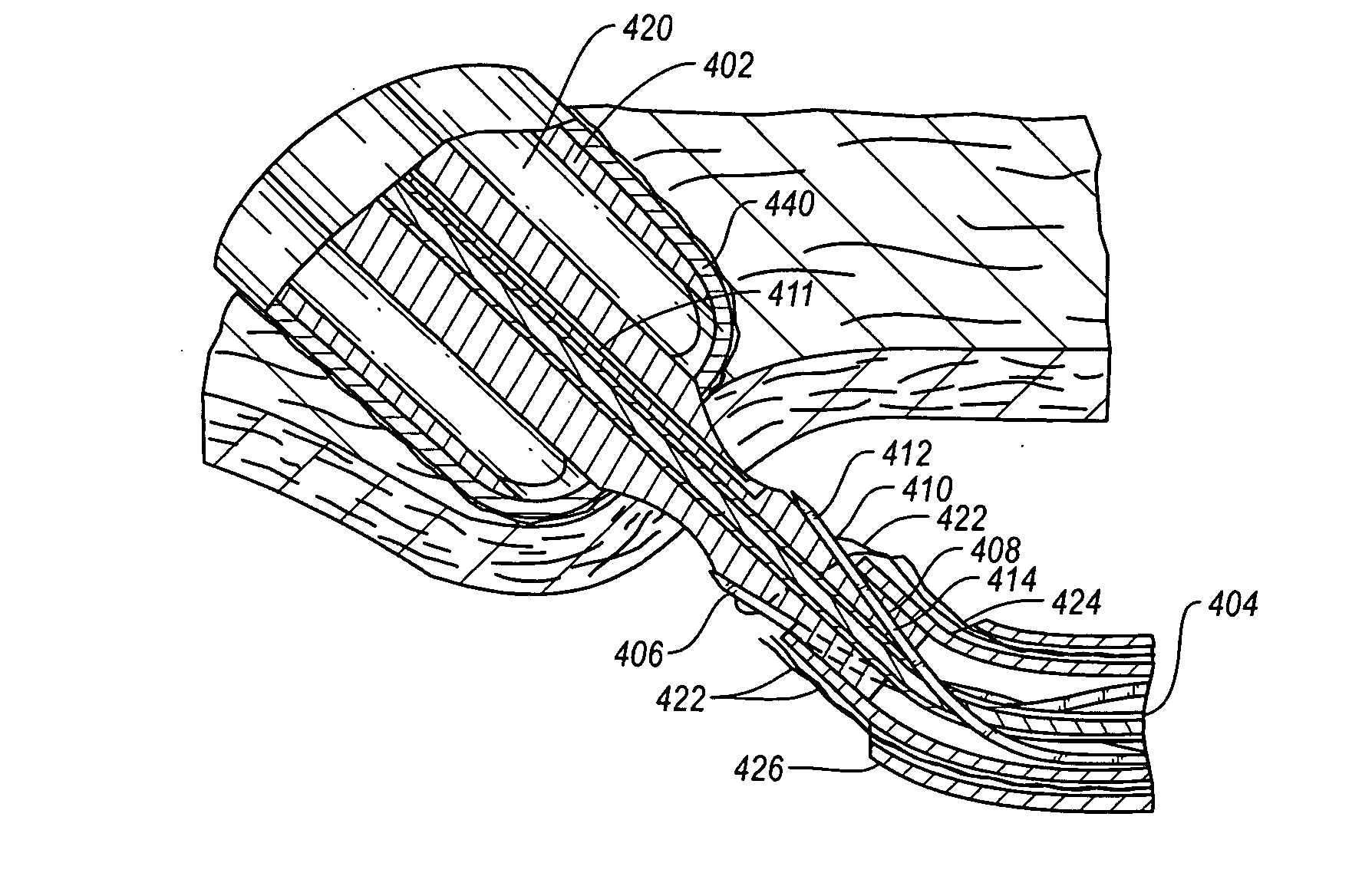
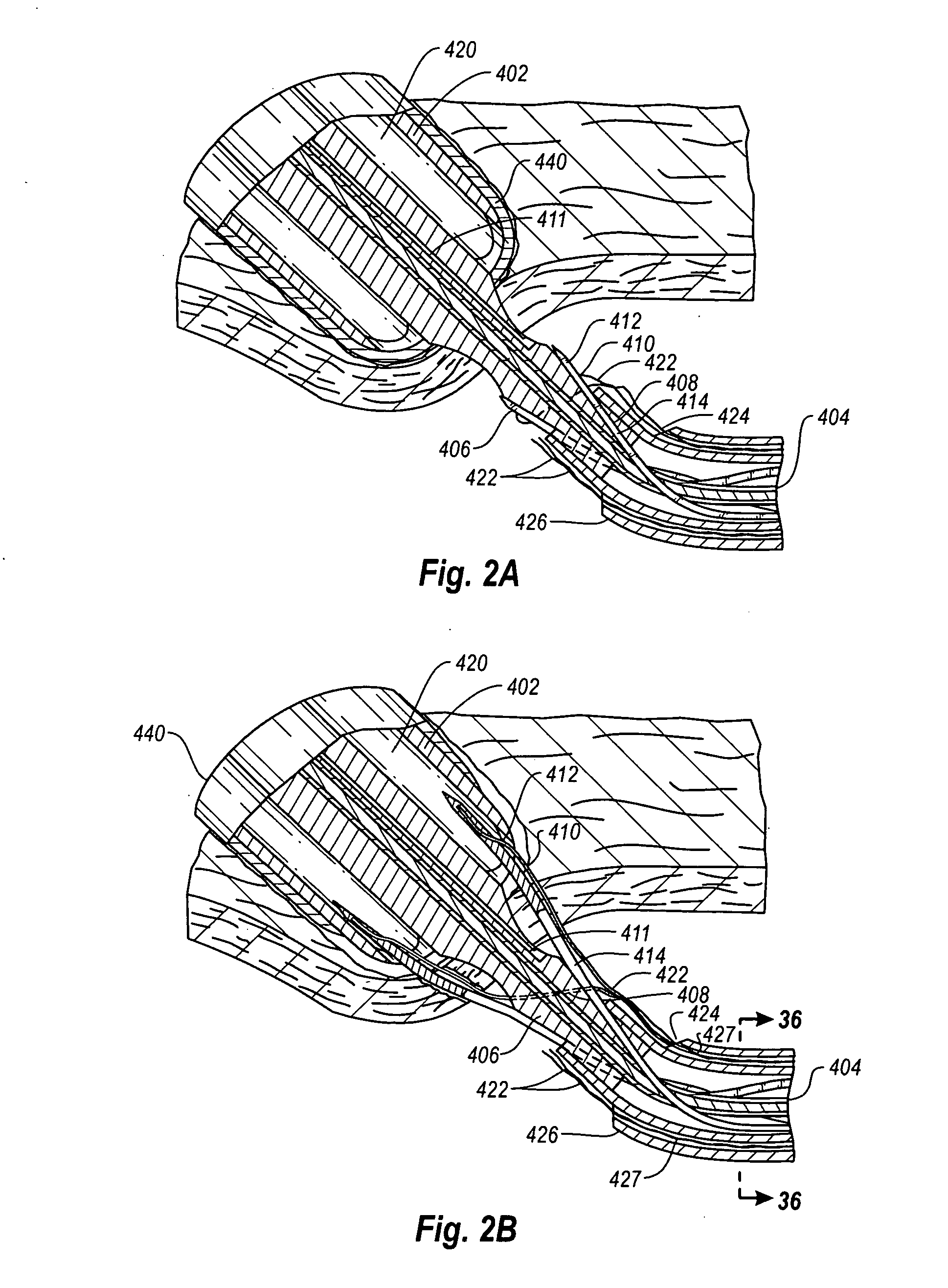
[0083]As used herein, the term “distal” is generally defined as in the direction of the patient, or away from a user of a device, or in a downstream direction relative to a forward flow of blood. In the context of a medical device intervention with or through a vessel wall, “distal” herein refers to the interior or the lumen side of the vessel wall.
[0084]Conversely, “proximal” generally means away from the patient, or toward the user, or in an upstream direction relative to a forward flow of blood. In the context of a medical device intervention with or through a vessel wall, “proximal” herein refers to the exterior or outer side of the vessel wall.
[0085]Additionally, “oblong” is herein intended to mean oval, elliptical, or otherwise having a generally rounded shape that is not perfectly circular. In particular, the term describes the shape of a tubular graft end cut at an acute angle relative to the plane perpendicular to the tissue walls defining the graft.
[0086]The term “hemostas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com