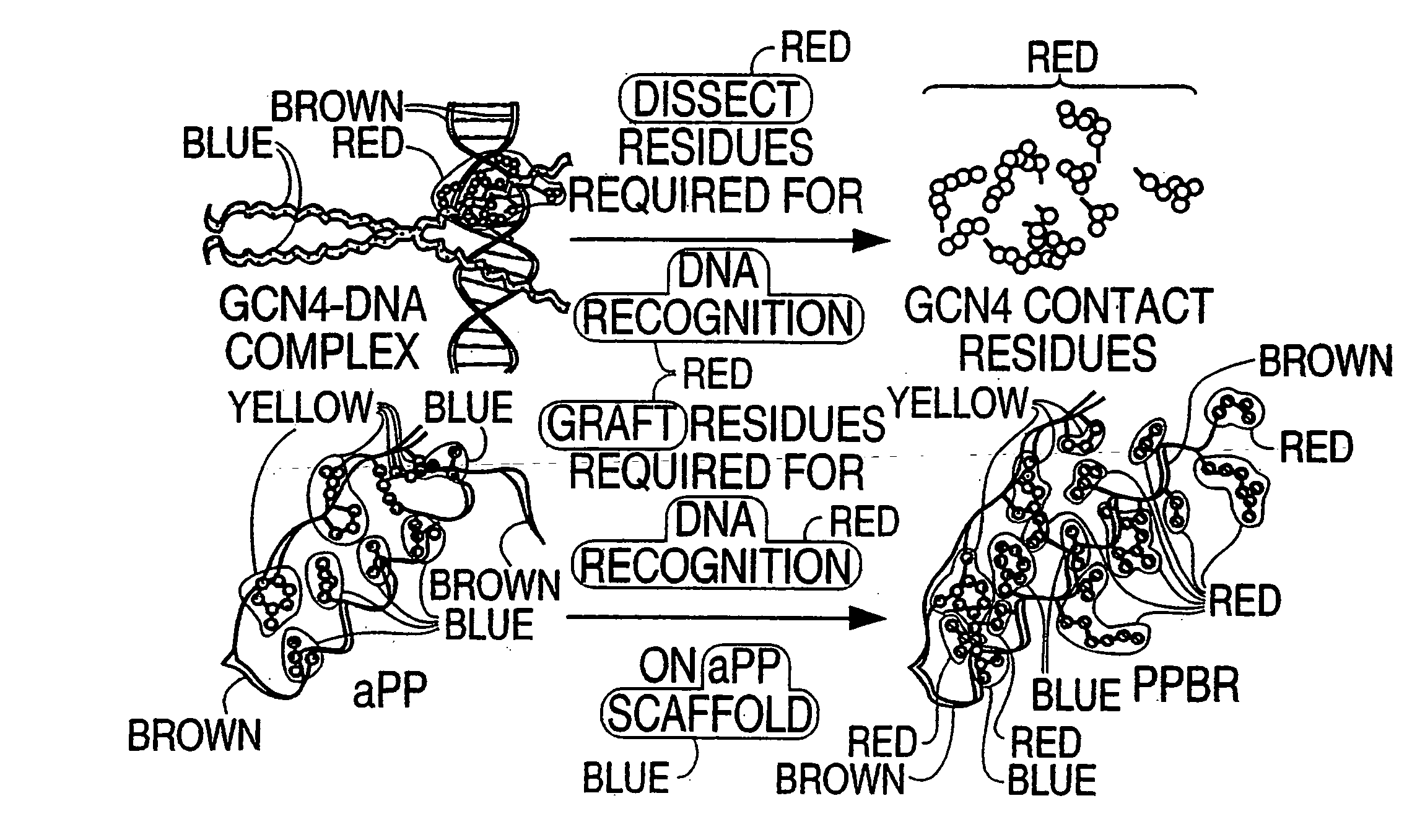
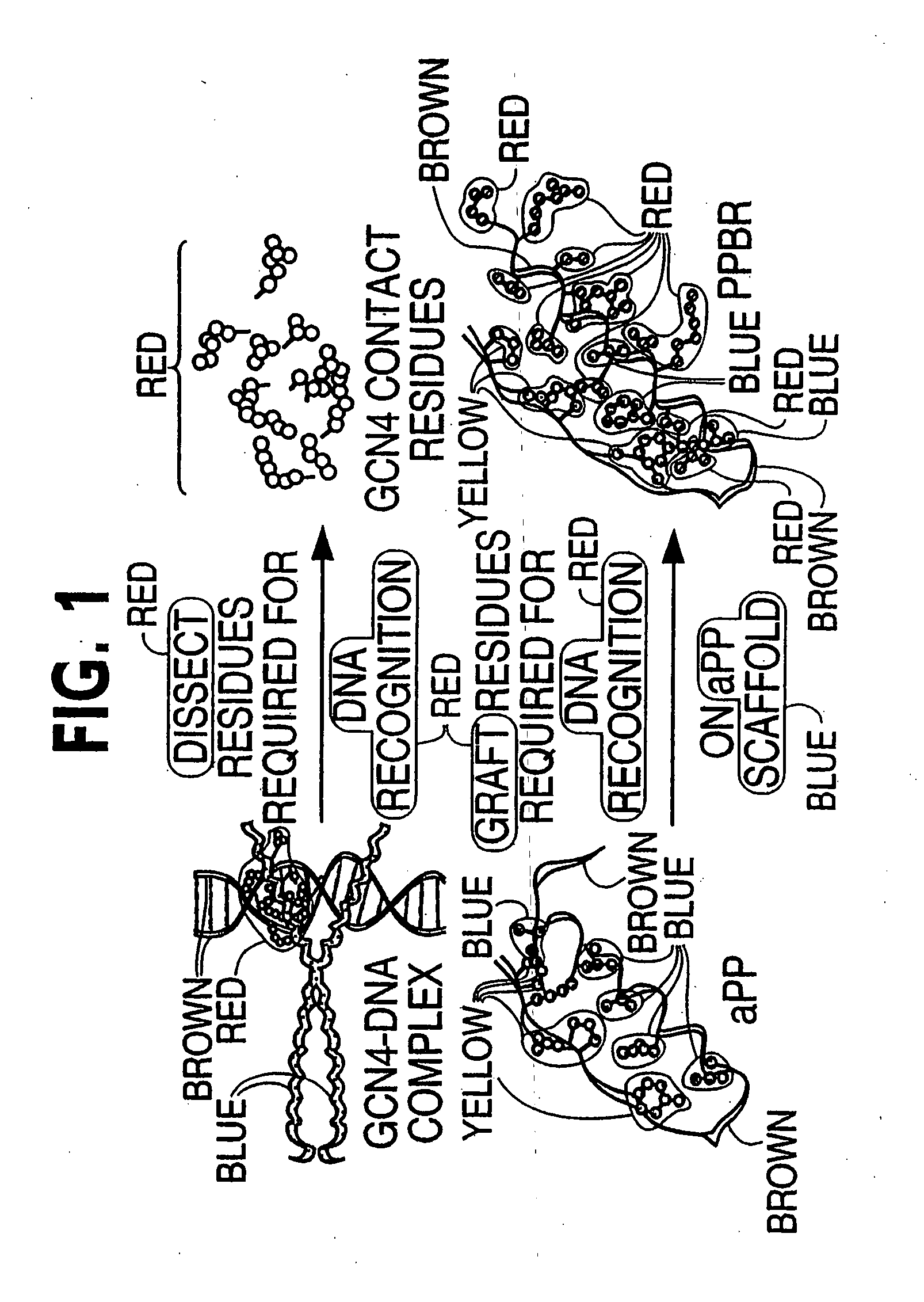
DNA & protein binding miniature proteins
a miniature protein and dna technology, applied in the field of polypeptide scaffolds, can solve the problems of difficult to envision a simple and general application of this truncation strategy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of DNA-Binding Miniature Proteins
[0082] Polypeptides constituting miniature proteins were prepared using solid phase methodology and contain a carboxy-terminal amide and a free amino terminus unless otherwise indicated. High performance liquid chromatography (HPLC) was performed on either a Waters 600E Multisolvent Delivery System with a Waters 490E multiwavelength detector or a Rainin Dynamax SD-200 Solvent Delivery System with a Rainin Dynamax PDA-2 Diode Array Detector.
[0083] Solid phase peptide synthesis was performed on a Perseptive BioSearch 9600 peptide synthesizer. Standard research grade argon (Connecticut AirGas) was passed through an OxyClear oxygen scrubber before introduction to the synthesizer. HATU (O-(7-benzotrizol-1-yl)-1,1,3,3,-tetramethyl uronium hexafluorophosphate) was used as the activating reagent without addition of supplemental benzotrizole. Dimethylformamide, piperidine and methylene chloride (Baker) were fresh and stored under nitrogen. Anhydro...
example 2
Binding of Miniature Proteins to DNA
[0092] Miniature protein-binding to DNA was measured using a electrophoretic mobility shift assay performed in a Model SE600 Dual-Controller Vertical Slab Unit (Hoefer) using 14×16 cm gel plates. Temperature was controlled using a constant temperature bath. Reactions were performed in a binding buffer composed of 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM NaH2PO4 (pH 7.4), 1 mM EDTA, 0.1% NP-40, 0.4 mg / ml BSA (non-acetylated) and 5% glycerol. For experiments involving the bZIP peptide C / EBP152, the binding buffer was supplemented with 2 mM dithiothreitol. Serial peptide dilutions were performed as 1:1 dilutions with binding buffer. In general, 0.002 ml of gamma 32P-labeled, double-stranded DNA (CRE24, hsCRE24, C / EBP24 or hsCEBP24; final concentration ≦50 pM in binding buffer; final concentration ≦5 pM for peptides with Kappapp / peptideTn))−1] where n=1 for PPEBPSR and EBPSR and n=2 for C / EBP152. In these equations, theta=cpm in protein-DNA co...
example 3
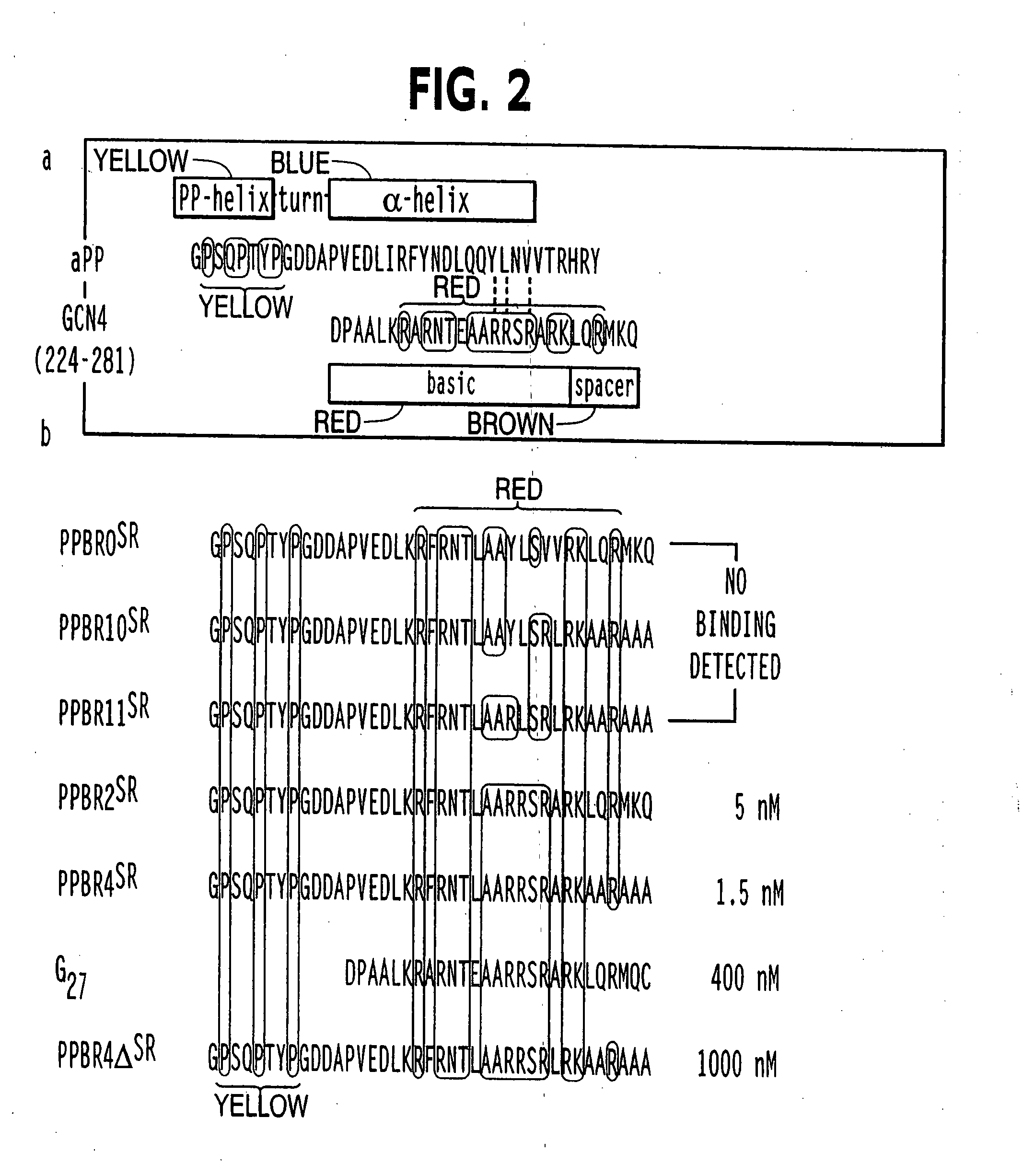
Role of Hydrophobic Core in Miniature Protein-Binding to DNA
[0096] The contribution of hydrophobic core formation on PPBR4SR-hsCRE24 complex stability was examined utilizing UV circular dichroism experiments. Circular dichroism spectra were recorded in PBS on an Aviv-202 CD spectrometer and were background corrected but not smoothed. Wavelength scans were performed at 4° C. between 200 and 260 nm at 1 nm intervals with a recording time of five seconds at each interval. Thermal denaturation curves were measured at 222 nm between 4° C. and 98° C. with 2° C. steps and one minute equilibration at each temperature. Mean residue ellipticity and percent helicity were calculated from the value at 222 nm after background correction.
[0097] G27 lacked the polyproline helix and turn, whereas PPBR4-deltaSR contained D-tryptophan at position four and leucine at position thirty-one. Modeling studies suggested that these substitutions would disrupt core formation by kinking the polyproline or the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com