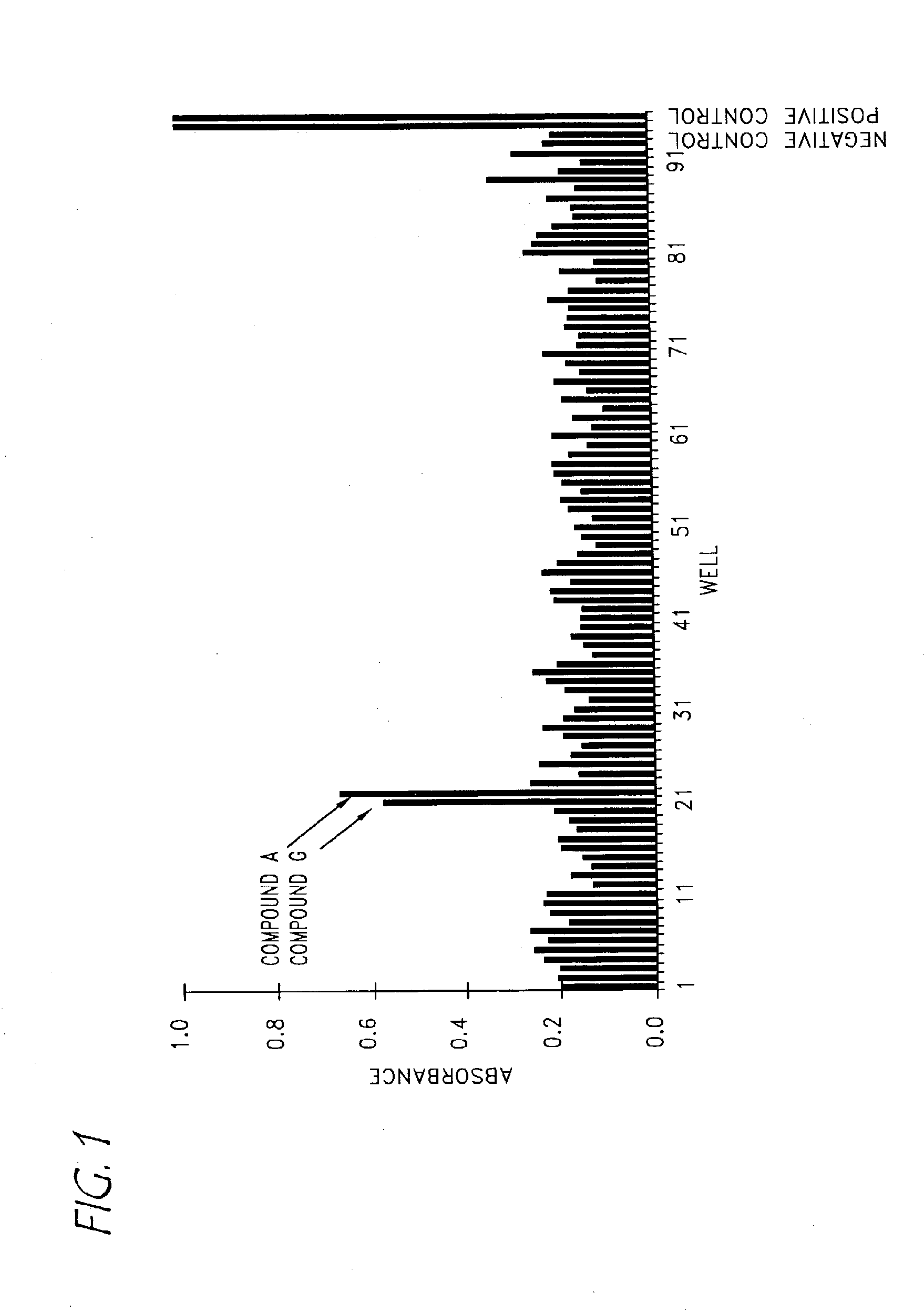
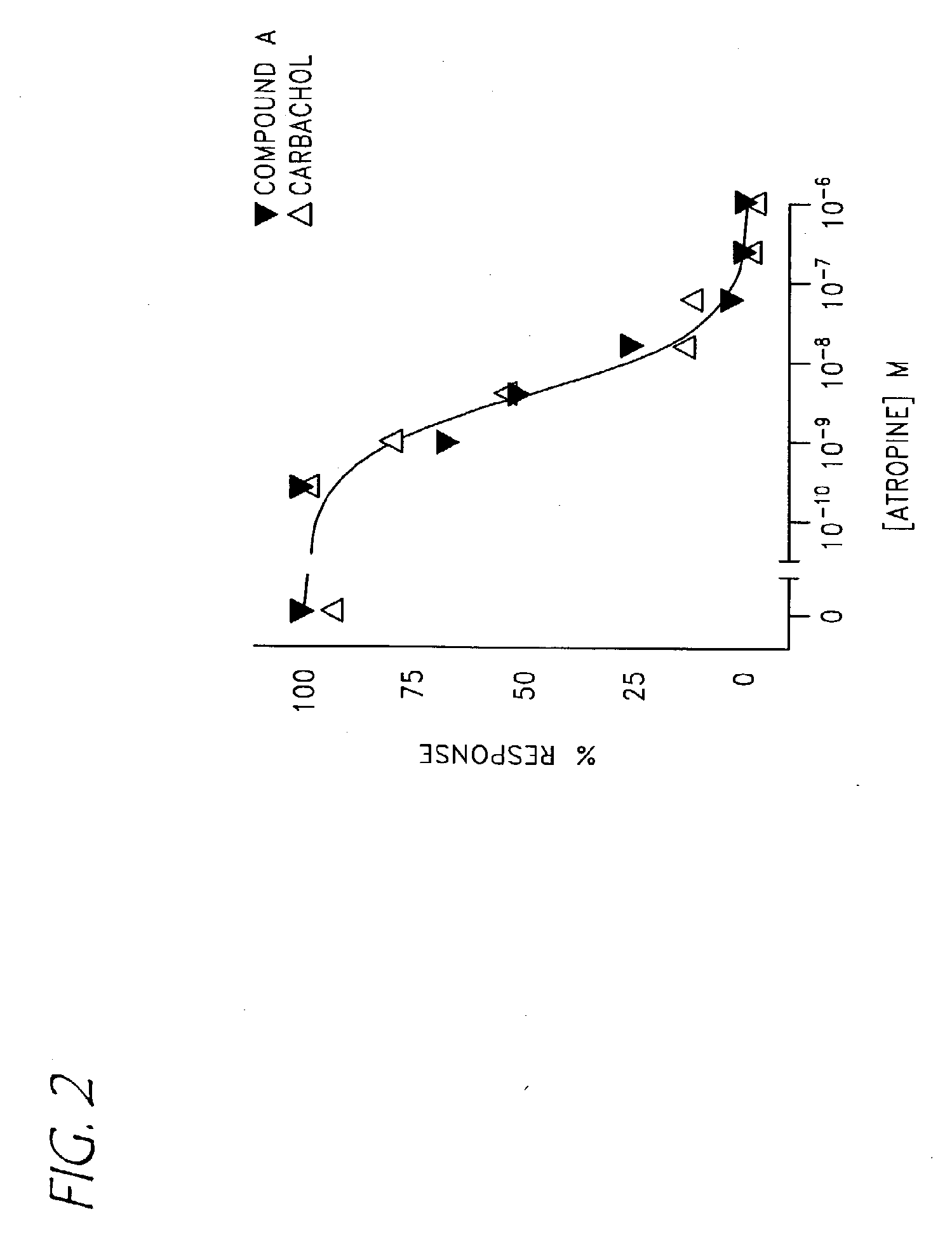
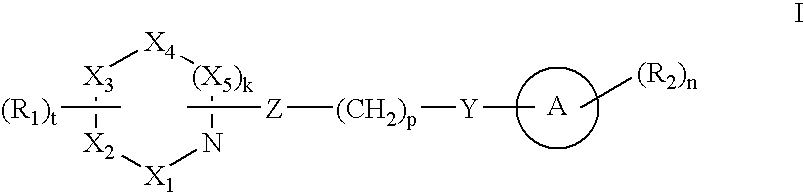Compounds with activity on muscarinic receptors
a technology of muscarinic receptors and compounds, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, biocides, etc., can solve the problems of limited clinical utility of tacrine, cholinergic side effects of tacrine, and limited doses of tacrin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
4-n-Butyl-1-[4-(2-methylphenyl)-4-oxo-1-butyl]piperidine (5)
[0193] 1-Benzyl-4-n-butylidenepiperidine (2). A 500 mL 3-necked flask fitted with a stirrer, was charged with sodium hydride (1.61 g, 67 mmol) and DMSO (40 mL). The resulting suspension was heated to 90° C. for 30 min, until the evolution of hydrogen ceased. The suspension was cooled on an ice-bath for 20 min followed by addition of a slurry of butyltriphenylphosphonium bromide (26.6 g, 67 mmol) in DMSO (70 mL). The red mixture was stirred for 15 min at rt. 1-Benzyl-4-piperidone 1 (14.0 g, 74 mmol) was slowly added over 30 min, and the mixture was stirred at room temperature over night. H2O (200 mL) was added to the reaction mixture followed by extraction with heptane (4×100 mL) and ethyl acetate (2×100 mL). The combined organic phases were dried and evaporated to dryness, producing 38.1 g of a yellow oil. The oil was distilled to give 14.9 g (88%) of 2, bp 101-105° C. (0.1 mm Hg). 1H NMR (CDCl3) δ 0.90-0.95 (t, 3H), 1.25-...
example ii
3-Hydroxymethyl-[4-(2-methylphenyl)-4-oxo-1-butyl]piperidine (7)
[0197] 4-(3-Hydroxymethyl-piperidin-1-yl)-butyronitrile (6). In an oven-dried 25 mL flask was placed piperidine-3-yl-methanol (1.12 g, 10 mmol) in acetonitrile (10 mL), followed by addition of potassium carbonate (1.38 g, 10 mmol) and 4-bromobutyronitrile (0.90 mL, 9 mmol). The reaction mixture was stirred at rt. for 12 h. The mixture was filtered and evaporated to dryness. Addition of H2O (20 mL) was followed by extraction with ethyl acetate (3×20 mL) and the combined organic phases were dried (MgSO4) and evaporated to dryness to produce 1.50 g of crude 6 which was used without further purification in the synthesis of compound 7.
[0198] 3-Hydroxymethyl-[4-(2-methylphenyl)-4-oxo-1-butyl]piperidine (7). In a 50 mL oven-dried flask was added Mg turnings (780 mg, 32 mmol), which were activated by the use of a heat-gun under vacuum, followed by addition of anhydrous THF (7 mL). Under inert atmosphere was added a suspension...
example iii
2-Propyl-[4-(2-methylphenyl)-4-oxo-1-butyl]piperidine (9)
[0199] 4-(2-propyl-piperidin-1-yl)-butyronitrile (8). A mixture of 2-propylpiperidine (550 mg, 4.3 mmol), 4-bromobutyronitrile (430 mg, 3.0 mmol) and potassium carbonate (550 mg, 4.0 mmol) in acetonitrile (5 mL) was stirred at rt for 12 h., followed by addition of a saturated brine (25 mL). The reaction mixture was extracted with ethyl acetate (3×25 mL) and the combined organic phases were dried (MgSO4) and evaporated to dryness to produce crude 8. The crude product was subjected to CC [eluent: CH2Cl2:MeOH (99:1)] to give pure 8 (0.48 g, 83%); LC-MS [M+H]+ 194 (cald. 194.2).
[0200] 2-Propyl-[4-(2-methylphenyl)-4-oxo-1-butyl]piperidine (9). In a 10 mL oven-dried flask was added Mg turnings (97 mg, 4.1 mmol) which were activated by the use of a heat-gun under vacuum. Under inert atmosphere was added a suspension of 2-iodotoluene (380 μl, 2.8 mmol) in Et2O (3 mL) and the reaction mixture was allowed to reflux for 1 hour. A mixtu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mol % | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com