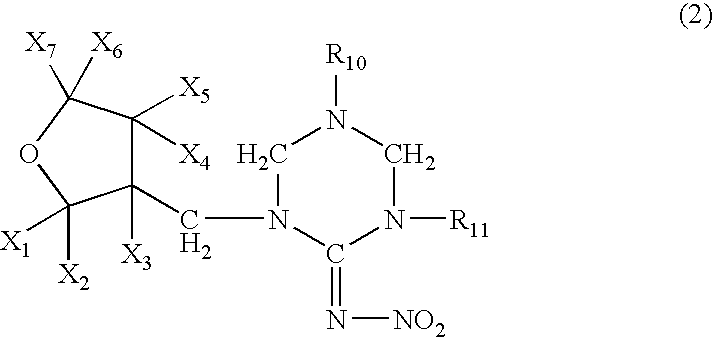
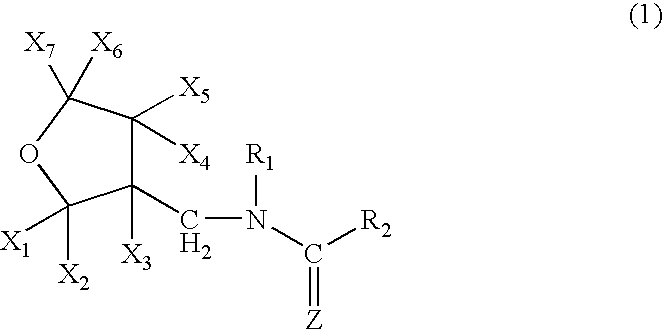High concentration dinotefuran formulations
a dinotefuran and formulation technology, applied in the field of insecticides, can solve the problems of reducing the efficacy of the solution applied to the animal, and achieve the effects of reducing the amount of insecticide, and reducing the efficacy of the solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 1-{(tetrahydro-3-furanyl)methyl}-2-nitro-3-methylguanidine (dinotefuran)
[0071] A mixture comprising 10.0 g of (tetrahydro-3-furanyl)methanol, 29.5 g of trifluoromethanesulfonic anhydride, 10.0 g of pyridine and 200 ml of dichloromethane was stirred for an hour at room temperature. Water was poured into the reaction solution to separate the organic layer, which was washed with 1 N hydrochloric acid, water and a saturated saline solution, dried, and concentrated to obtain 20 g of 3-tetrahydro-furanylmethyl triflate. 3.25 g of 60% sodium hydride were added to 12.5 g of 1,5-dimethyl-2-nitroiminohexahydro-1,3,5-triazine and 60 ml of DMF at room temperature, followed by stirring for an hour. 20.0 g of the 3-tetrahydrofuranylmethyl triflate were added thereto, and the mixture was stirred at 50° C. for 2 hours. After cooling the mixture to room temperature, 50 ml of 2N hydrochloric acid were added thereto, followed by stirring at 50° C. for 2 hours. The resultant mixture was...
example 2
Preparation of Insecticide Formulation Containing Dinotefuran, Ethanol and Water
[0072] 5 g (i.e., 5.6% (weight / weight)) of dinotefuran was dissolved into 100 ml of a mixture comprising 70% ethanol and 30% water. The resulting mixture can be spot applied to companion animals, such as dogs and cats and will kill fleas, ticks and other insects.
example 3
Preparation of Insecticide Formulation Containing Dinotefuran and Phenyl Methanol
[0073] 15 g (i.e., 12.5% (weight / weight)) of dinotefuran was dissolved into 100 ml of phenyl methanol. The resulting solution can be spot applied to companion animals, such as dogs and cats and will kill fleas, ticks and other insects.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com