Antibodies as a cancer diagnostic
a cancer and antibody technology, applied in the field of metastatic disease diagnosis, can solve the problems of little known about epha2 function, and achieve the effects of reducing cell-cell contact, increasing interaction, and reducing cell adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
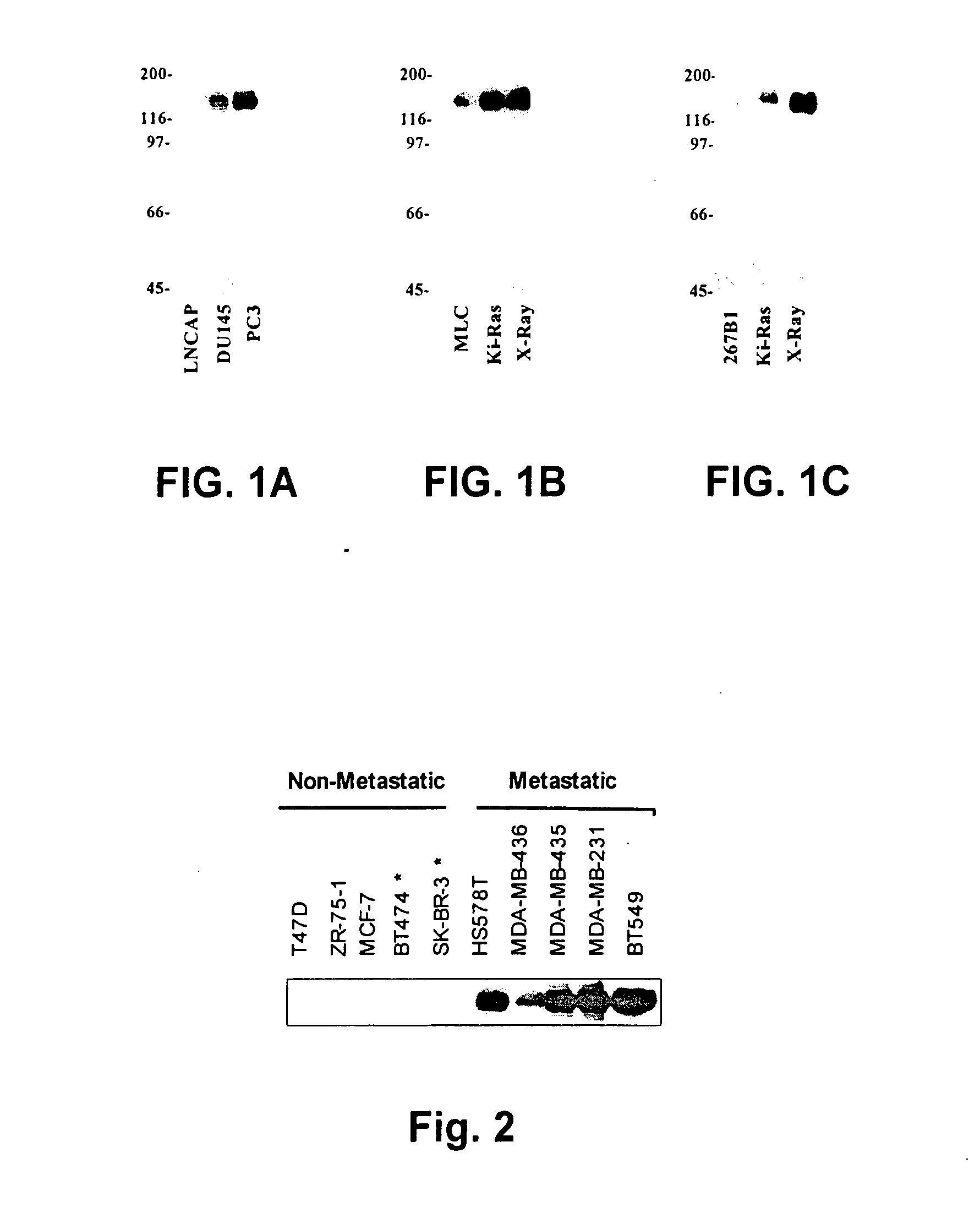
[0028] The antibodies produced by the D7 hybridoma are used to detect differential expression of EphA2 between normal prostate epithelial cells and metastatic cells. FIG. 1 shows EphA2 expression in various human prostate cell lines. Referring first to FIG. 1A, three metastatic cell lines, LNCAP, DU145, and PC3, are tested for levels of EphA2 expression. It is known that, of these three cell lines, LNCAP is the least invasive, DU145 is somewhat more invasive, and PC3 is the most invasive. EphA2 expression is determined by western blotting with D7 antibodies. As can be seen in FIG. 1A, EphA2 expression positively correlates with invasiveness.
[0029] In FIG. 1B, D7 antibodies are used to test EphA2 expression in normal MLC cells as compared to expression in transformed cells. Normal MLC cells, MLC cells which have been transformed by K-Ras, and MLC cells with have been transformed by X-irradiation are studied. As can be seen in FIG. 1B, EphA2 is overexpressed in both of the transforme...
example 2
[0030] EphA2 antibodies are used to detect altered EphA2 expression in metastatic mammary cells. EphA2 is expressed in normal mammary epithelial cells. FIG. 2 illustrates altered EphA2 expression in mammary tumor cell lines. As can be seen in FIG. 2, western blots from whole cell lysates using D7 antibodies reveal that EphA2 expression is completely absent in cells derived from non-metastatic breast tumors (ZR75-1, BT474, SKBR3, MDA-MB-435). By contrast, EphA2 is overexpressed in metastatic breast cancer cell lines (MDA-MB-435, MDA-MB-231). Thus, EphA2 antibodies detect altered EphA2 expression in breast cancer cells, which can be used to diagnose metastasis. Moreover, in non-metastatic breast epithelial cells, loss of EphA2 occurs early in the disease, and testing with EphA2-specific antibodies provide information relevant to invasiveness even when other known markers remain normal. Thus, D7 antibodies are useful as a diagnostic, even in early stages of disease.
example 3
[0031] EphA2 antibodies in combination wvitlh other antibodies are used to detect further alterations in EphA2 expression. As discussed above in Example 2, wvesterni blots using D7 can distinguish between non-metastatic and metastatic tumors, with non-metastatic tumors failing to express EphA2, and metastatic cells overexpressing EphA2. However, different results are found when tyrosine phosphorylation is studied. Using a phosphotyrosine-specific antibody, it has been found that EphA2 is phosphorylated in normal cells, but it is not phosphorylated in metastatic cells. Thus, while EphA2 specific antibodies can qualitatively detect a difference between metastatic and non-metastatic mammary tumor cells, diagnostics incorporating both an EphA2-specific antibody and a phosphotyrosine-specific antibody provides a sensitive test for distinguishing between normal, non-metastatic, and metastatic mammary cells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescent | aaaaa | aaaaa |
| physical interactions | aaaaa | aaaaa |
| cell adhesion | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com