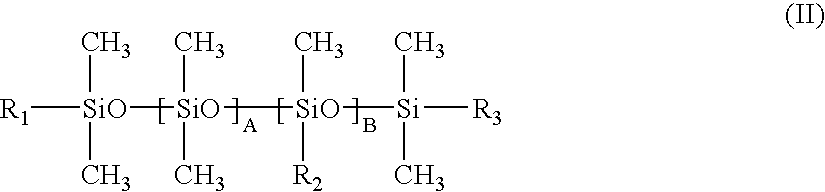
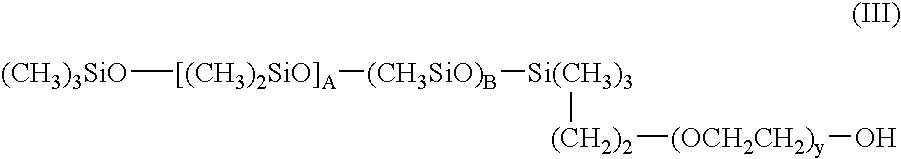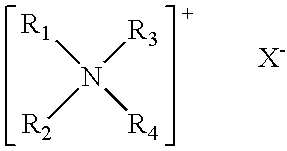Double-stranded RNA oligonucleotides which inhibit tyrosinase expression
a tyrosinase and oligonucleotide technology, applied in the field of double-stranded rna oligonucleotides, can solve the problems of inability to demonstrate the effectiveness of sirnas homologous to these anti-sense rnas, and the use of sirnas in vivo is known to present various difficulties, and achieves the effect of permanent depigmentation of the skin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ons
[0297] Cationic Micelles:
[0298] Exemplar 1: Octyl-p-glucoside / cetyltriammonium bromide at the Molar Ratio of 5 / 1:
E1: 100 mM in distilled water
E2: 50 mM in distilled water
E3: 25 mM in distilled water
E4: 12.5 mM in distilled water.
[0299] The 2 surfactants (nonionic+cationic) are solubilized in distilled water. A suspension of the siRNA of SEQ ID NO. 1 (20 μM) is added at from 1:1 and 1:9 volume (siRNA: micelles), thus reducing the micellar concentration in proportion.
[0300] Exemplar 2: Micelles of decyl-β-glucoside / behenyltriammonium chloride at the molar ratio of 5 / 1 in distilled water. The same concentrations as in the preceding exemplar are prepared.
[0301] These 2 suspensions can be applied to the skin for the treatment of pigmentation marks and dyschromia or for the bleaching of hair follicles.
[0302] Cationic liposomes:
[0303] Exemplar 1: “Fluid” Vesicles:
PEG 400 isostearate5.5%Behenyltriammonium chloride0.5%Distilled waterqs 100
[0304] Exemplar 2: “Rigid” Vesicle...
example 2
nt of the Activity of the siRNAs According to the Invention
[0325] The effectiveness of the 47 siRNAs of sequence SEQ ID NOS 1 to 47 according to the invention was evaluated in the “pSCREEN-iT™ system” test developed by Invitrogen, set up to evaluate the effectiveness on tyrosinase inhibition (GenBank accession number M27160).
[0326] Experimental protocol for the test: [0327] 2×104 A549 cells / well are seeded into a 48-well plate and cultured overnight. [0328] Cotransfection is carried out in triplicate for 5 h using 2 μg / ml of Lipofectamine 2000 (Invitrogen) complexed with: [0329] tyrosinase pScreen-iT™ vector coupled to the LacZ gene—10 ng / well [0330] luciferase reporter plasmid—20 ng / well [0331] 1 nM Stealth™ RNA (sequences given in Table I) [0332] The cells are lysed 24 h after the transfection and the β-galactosidase (β-Gal) and luciferase (Luc) activities are measured. The β-Gal / Luc ratio determines the percentage inhibition of tyrosinase.
[0333] The results obtained are reporte...
example 3
n of a Possible Interferon-Type Response Caused by the siRNAs According to the Invention
[0336] It was verified that the siRNAs which are subjects of the present invention did not induce an interferon-type response. For this, the expression of the OAS-1 and IFIT-1 (interferon-inducible tetratricopeptide repeat domain) genes, known to be induced by interferon (Wieland S F et al., Searching for Interferon-induced Genes That Inhibit Hepatitis B Virus Replication in Transgenic Mouse Hepatocytes, J. of Virology 2003; 77: 1227-1236. Persengiev S P et al., Nonspecific, concentration-dependent stimulation and repression of mammalian gene expression by small interfering RNAs (siRNAs). RNA 2004; 10: 12-18. Bridge A J et al., Induction of an interferon response by RNAi vectors in mammalian cells. Nature Genet. 2003; 34: 263-264. Scacheri P C et al., Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. Proc. Natl. Acad. Sci. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| zeta potential | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com