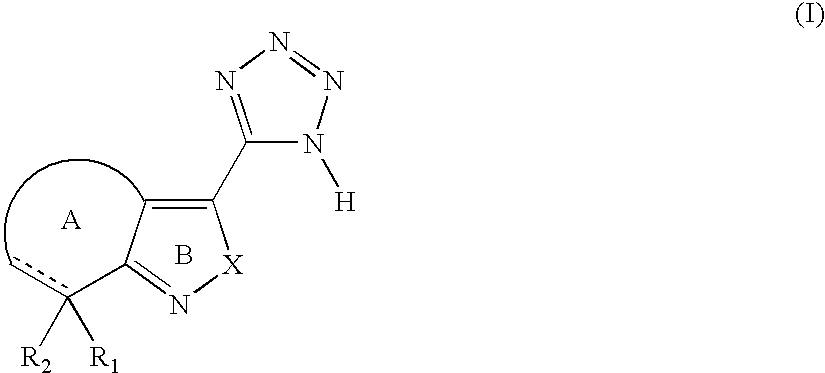
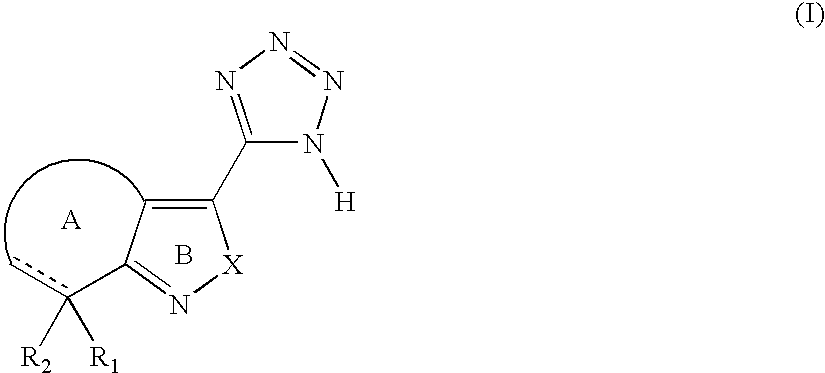
Tetrazole derivatives and methods of treatment of metabolic-related disorders thereof
a technology of tetrazolium derivatives and metabolic disorders, applied in the field of tetrazolium derivatives and methods of treatment of metabolic disorders thereof, can solve the problems of liver toxicity, large and increasing use of nicotinic acid as a therapeutic agent, and serious public health problems such as type 2 diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Rodent Diabetes Models
[0253] Rodent models of type 2 diabetes associated with obesity and insulin resistance have been developed. Genetic models such as db / db and ob / ob [see Diabetes (1982) 31:1-6] in mice and fa / fa in zucker rats have been developed for understanding the pathophysiology of disease and for testing candidate therapeutic compounds [Diabetes (1983) 32:830-838; Annu Rep Sankyo Res Lab (1994) 46:1-57]. The homozygous animals, C57 BL / KsJ-db / db mice developed by Jackson Laboratory are obese, hyperglycemic, hyperinsulinemic and insulin resistant [J Clin Invest (1990) 85:962-967], whereas heterozygotes are lean and normoglycemic. In the db / db model, mice progressively develop insulinopenia with age, a feature commonly observed in late stages of human type 2 diabetes when sugar levels are insufficiently controlled. Since this model resembles that of human type 2 diabetes, the compounds of the present invention are tested for activities including, but not limited to, lowering...
example 2
Mouse Atherosclerosis Model
[0256] Adiponectin-deficient mice generated through knocking out the adiponectin gene have been shown to be predisposed to atherosclerosis and to be insulin resistant. The mice are also a suitable model for ischemic heart disease [Matsuda, M et al. J Biol Chem (2002) July, and references cited therein, the disclosures of which are incorporated herein by reference in their entirety].
[0257] Adiponectin knockout mice are housed (7-9 mice / cage) under standard laboratory conditions at 22° C. and 50% relative humidity. The mice are dosed by micro-osmotic pumps, inserted using isoflurane anesthesia, to provide compounds of the invention, saline, or an irrelevant compound to the mice subcutaneously (s.c.). Neointimal thickening and ischemic heart disease are determined for different groups of mice sacrificed at different time intervals. Significant differences between groups (comparing compounds of the invention to saline-treated) are evaluated using Student t-t...
example 3
[0258] A modified Flash Plate™ Adenylyl Cyclase kit (New England Nuclear; Cat. No. SMP004A) was utilized for direct identification of candidate compounds as agonists to hRUP25 in accordance with the following protocol. The term hRUP25 includes the human sequences found in GenBank Accession No. NM—177551 for the nucleotide and GenBank Accession No. NP 808219 for the polypeptide, and naturally-occurring allelic variants, mammalian orthologs, and recombinant mutants thereof.
[0259] CHO cells stably transfected with an expression vector encoding hRUP25 and cultured under condition permissive for cell surface expression of the encoded hRUP25 receptor were harvested from flasks via non-enzymatic means. The cells were washed in PBS and resuspended in the manufacturer's Assay Buffer. Live cells were counted using a hemacytometer and Trypan blue exclusion, and the cell concentration was adjusted to 2×106 cells / ml. cAMP standards and Detection Buffer (comprising 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com