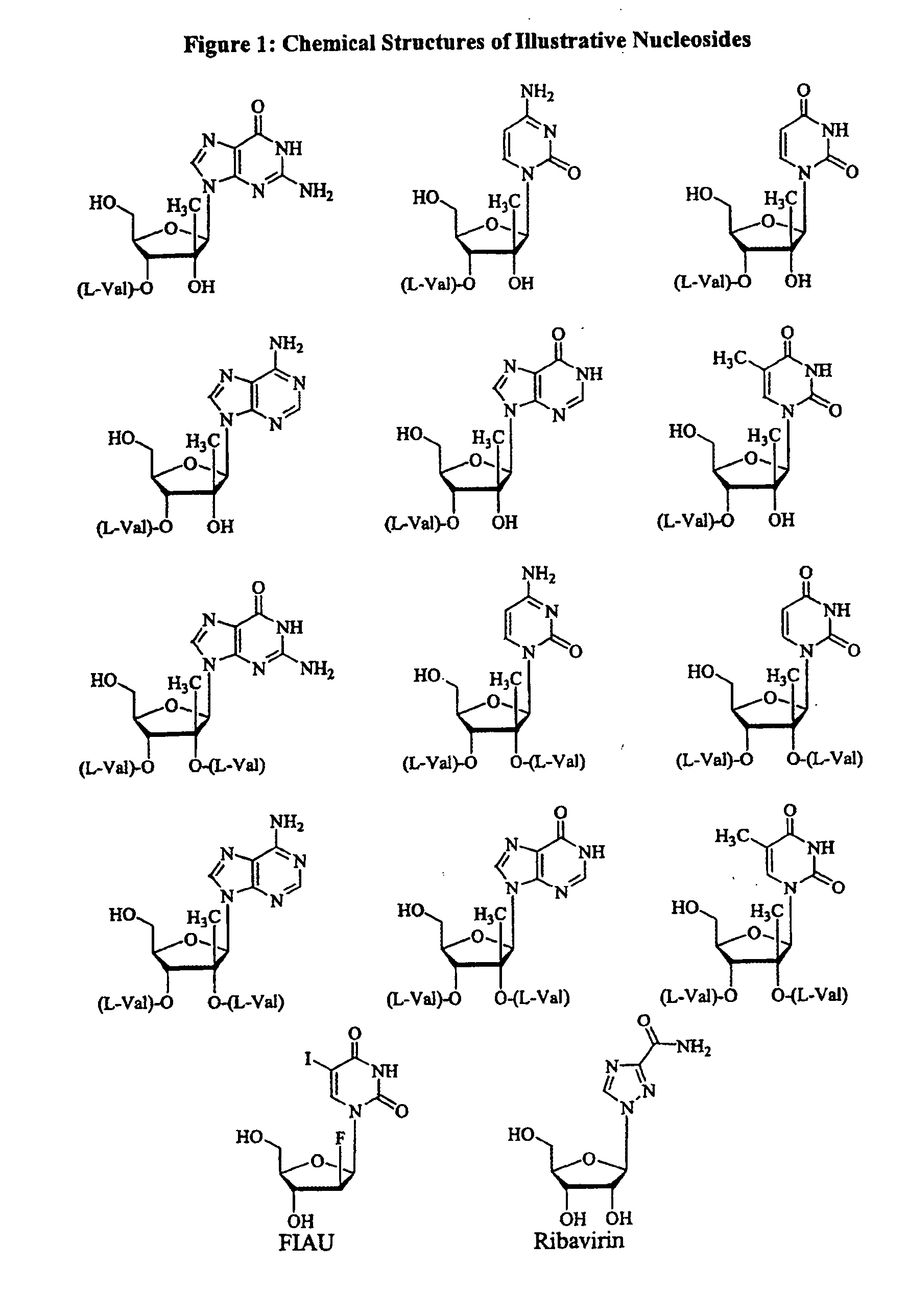
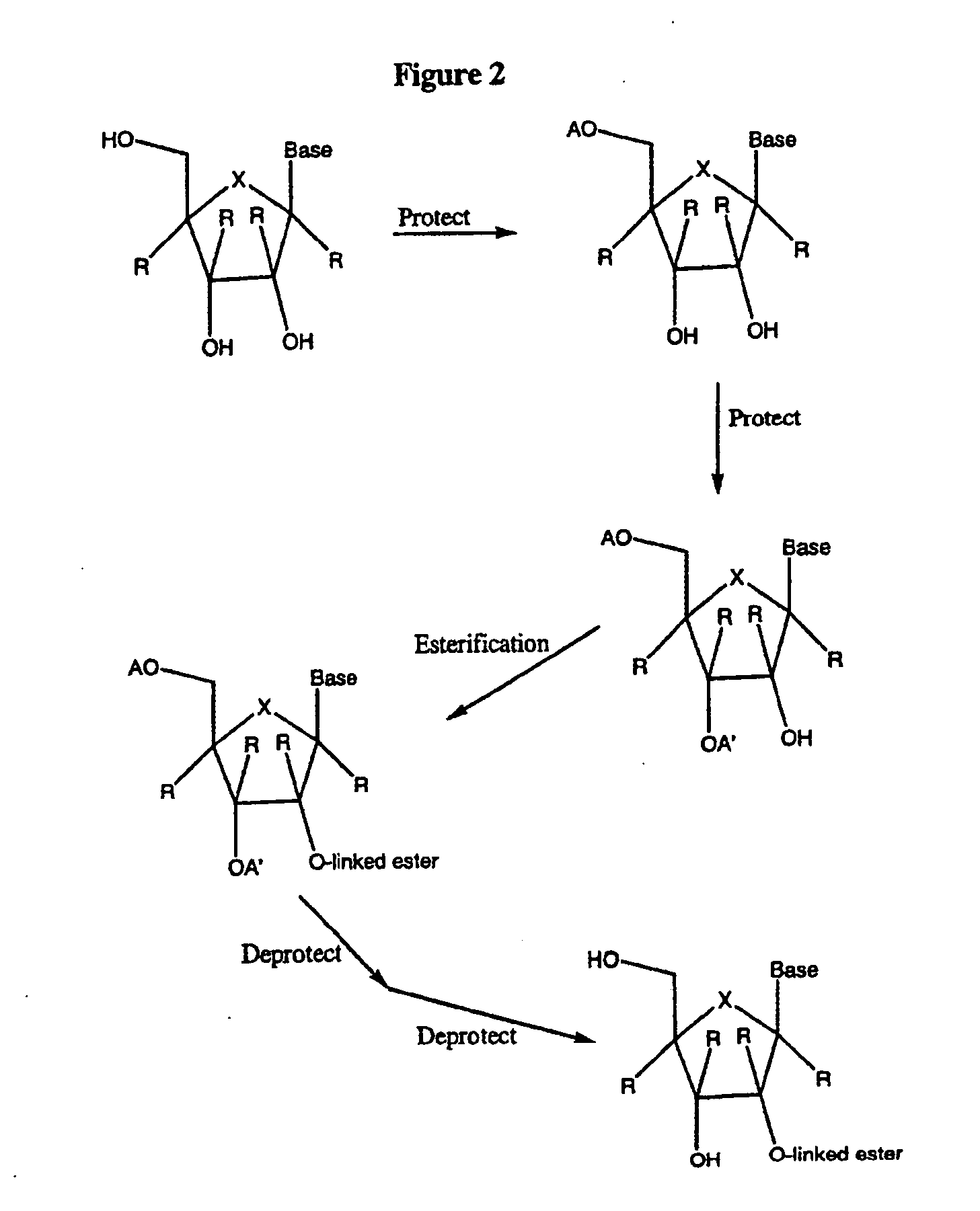
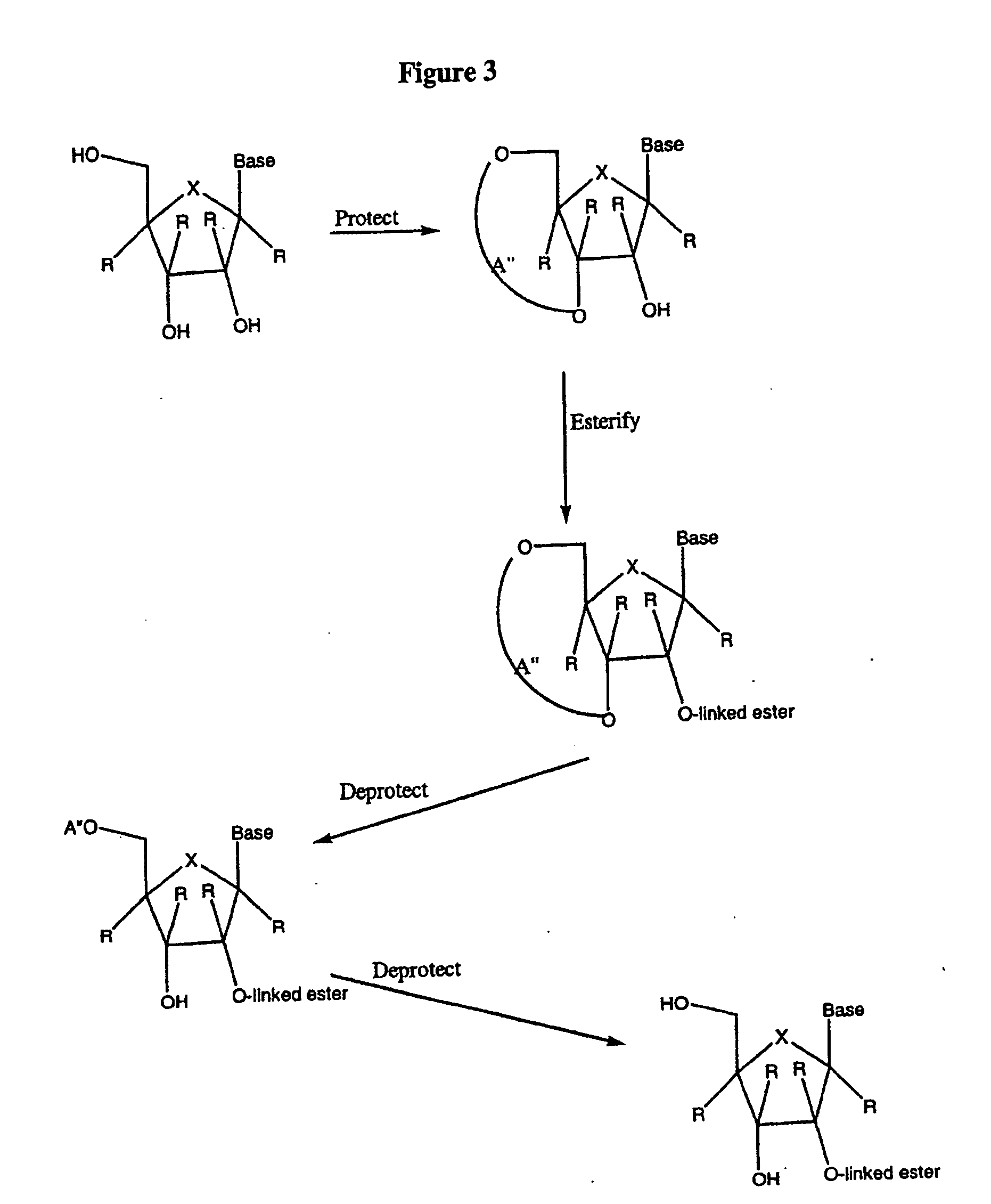
2' and 3'-nucleoside prodrugs for treating Flaviviridae infections
a technology of flaviviridae and nucleosides, applied in the field of pharmaceutical chemistry, can solve the problems of significant economic loss worldwide, severe flu-like symptoms, and significant pestivirus exposure, and achieve the effect of determining the activity spectrum and inhibiting the activity of hcv polymeras
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 1′-C-methylriboadenine via 6-amino-9-(1-deoxy-β-D-psicofuranosyl)purine
[0444] Melting points were determined on a Mel-temp II apparatus and are uncorrected. NMR spectra were recorded on a Bruker 400 AMX spectrometer at 400 MHz for 1H NMR and 100 MHz for 13C NMR with TMS as internal standard. Chemical shifts (δ) are reported in parts per million (ppm), and signals are reported as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), or bs (broad singlet). IR spectra were measured on a Nicolet 510P FT-IR spectrometer. Mass spectra were recorded on a Micromass Autospec high-resolution mass spectrometer. TLC were performed on Uniplates (silica gel) purchased from Analtech Co. Column chromatography was performed using either silica gel-60 (220-440 mesh) for flash chromatography or silica gel G (TLC grade, >440 mesh) for vacuum flash column chromatography. UV spectra were obtained on a Beckman DU 650 spectrophotometer. Elemental analysis was performed by Atlan...
example 2
Preparation of 2′-C-methylriboadenine
[0449] The title compound was prepared according to a published procedure (R. E. Harry-O'kuru, J. M. Smith, and M. S. Wolfe, “A short, flexible route toward 2′-C-branched ribonucleosides”, J. Org. Chem. 1997, 62, 1754-1759) (Scheme 11). [0450] (a) Dess-Martin periodinane; (b) MeMgBr / TiCl4; (c) BzCl, DMAP, Et3N; (d) bis(trimethylsilyl)acetamide, N6-benzoyl adenine, TMSOTf; (e) NH3 / MeOH
[0451] The 3′-prodrug of the 2′-branched nucleoside was prepared according to published procedure (Synthetic Communications, 1978, 8(5), 327-333; J. Am. Chem. Soc., 1999, 121(24), 5661-5664). Alternatively, the 2′-branched nucleoside can be esterified without protection (Scheme 11b). Carbonyldiimidazole (377 mg, 2.33 mmol) was added to a solution of N-(tert-butoxycarbonyl)-L-valine (507 mg, 2.33 mmol) in 15 mL of anhydrous tetrahydrofuran. The mixture was stirred at 20° C. for one hour and at 50° C. for 10 minutes and then added to a solution of 4-Amino-1-(3,4-dih...
example 3
Preparation of 3′-C-methylriboadenine
[0461] The title compound can be prepared according to a published procedure (R. F. Nutt, M. J. Dickinson, F. W. Holly, and E. Walton, “Branched-chain sugar nucleosides. III. 3′-C-methyladenine”, J. Org. Chem. 1968, 33, 1789-1795) (Scheme 12).
(a) RuO2 / NaIO4; (b) MeMgI / TiCl4; (c) HCl / MeOH / H2O; (d) BzCl / pyridine; (e) AcBr, HBr / AcOH; (f) chloromercuri-6-benzamidopurine; (g) NH3 / MeOH.
[0462] In a similar manner, but using the appropriate sugar and pyrimidine or purine bases, the following nucleosides of Formula III are prepared.
wherein R1, R2, R3, X1, X2, and Y are defined in Table 13.
[0463] Alternatively, the following nucleosides of Formula VI are prepared, using the appropriate sugar and pyrimidine or purine bases.
wherein R1, R2, R3, X1, and Y are defined in Table 14.
[0464] Alternatively, the following nucleosides of Formula XI are prepared, using the appropriate sugar and pyrimidine or purine bases.
wherein R1, R2, R3, R6, X, and Ba...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| reaction temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com