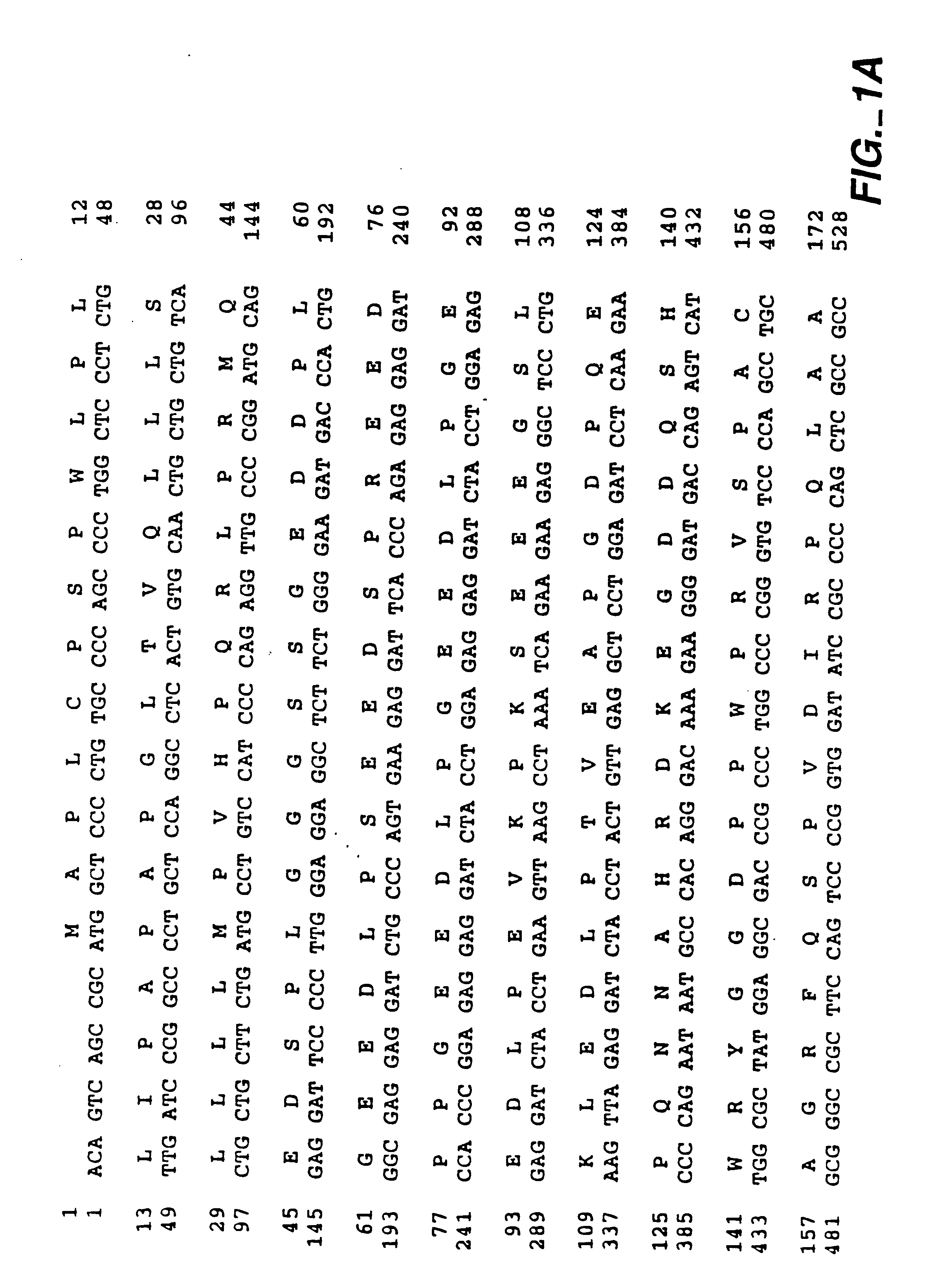
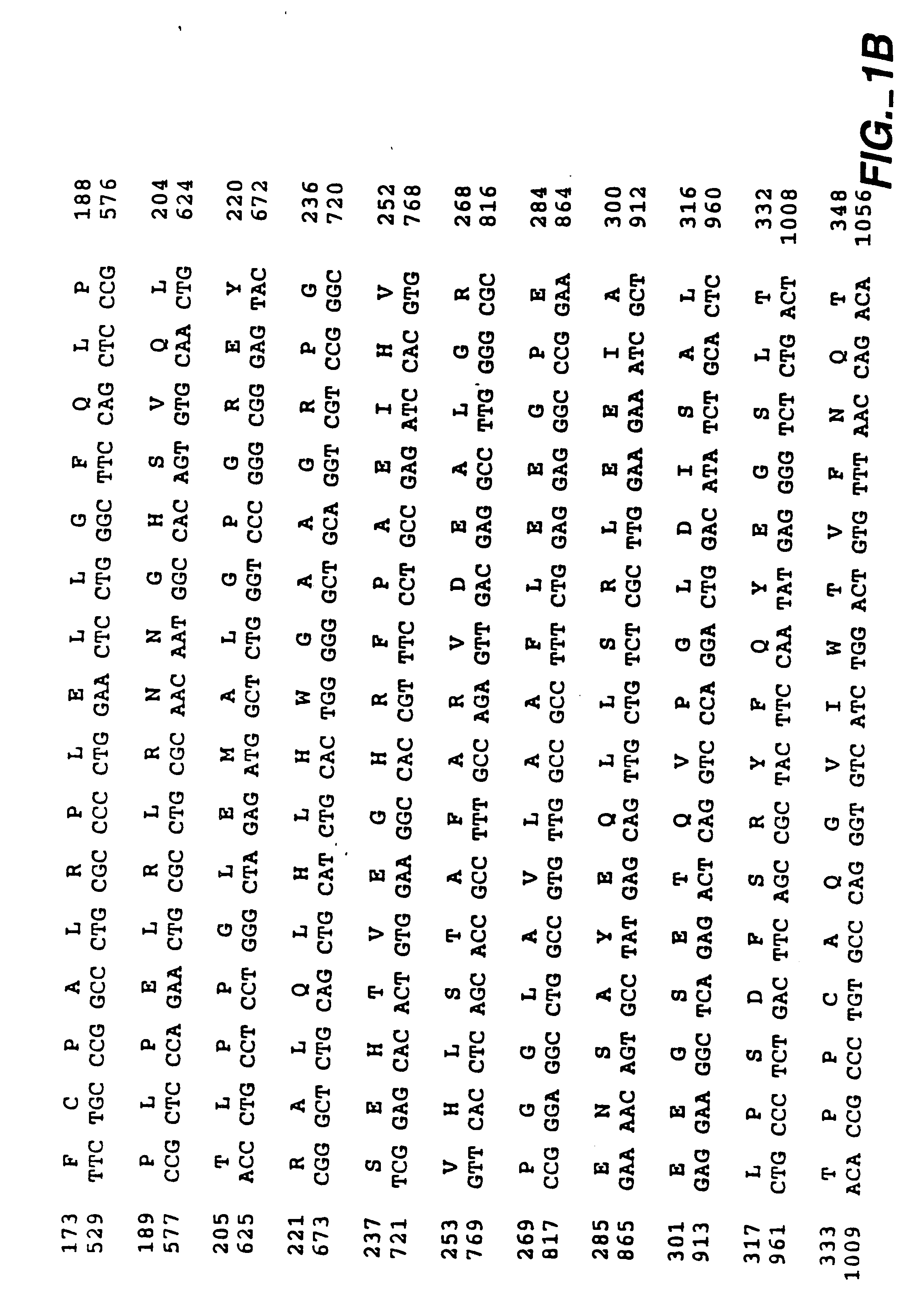
MN gene and protein
a technology of mn gene and protein, applied in the field of medical genetics, can solve the problems of not even one single stably transformed cell clone, disruption of the normal program of cell differentiation, and upset the correct communication among cells, so as to prevent recidivism and/or metastasis, inhibit the expression of mn genes, and prevent tumor recurrence.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Immunohistochemical Staining of Tissue Specimens
[0440] To study and evaluate the tissue distribution range and expression of MN proteins, the monoclonal antibody M75 was used to stain immunohistochemically a variety of human tissue specimens. The primary antibody used in these immunohistochemical staining experiments was the M75 monoclonal antibody. A biotinylated second antibody and streptavidin-peroxidase were used to detect the M75 reactivity in sections of formalin-fixed, paraffin-embedded tissue samples. A commercially available amplification kit, specifically the DAKO LSAB™ kit [DAKO Corp., Carpinteria, Calif. (USA)] which provides matched, ready made blocking reagent, secondary antibody and steptavidin-horseradish peroxidase was used in these experiments.
[0441] M75 immunoreactivity was tested according to the methods of this invention in multiple-tissue sections of breast, colon, cervical, lung and normal tissues. Such multiple-tissue sections were cut from paraffin blocks ...
example 2
Vaccine—Rat Model
[0455] As shown in Example 7 of WO 93 / 18152 (International Publication Date: 16 Sep. 1993), in some rat tumors, for example, the XC tumor cell line (cells from a rat rhabdomyosarcoma), a rat MN protein, related to human MN, is expressed. Thus a model was afforded to study antitumor immunity induced by experimental MN-based vaccines. The following representative experiments were performed.
[0456] Nine- to eleven-day-old Wistar rats from several families were randomized, injected intraperitoneally with 0.1 ml of either control rat sera (the C group) or with rat serum against the MN fusion protein GST-MN (the IM group). Simultaneously both groups were injected subcutaneously with 106 XC tumor cells.
[0457] Four weeks later, the rats were sacrificed, and their tumors weighed. The results indicated that the difference between the two groups—C and IM—was significant by Mann-Whitney rank test (U=84, α<0.025). The IM group of baby rats developed tumors about one-half the s...
example 3
Transient Transformation of Mammalian Cells by MN Protein
[0458] This example (1) examines the biological consequences of transfecting human or mouse cells with MN-cDNA inserted into expression vectors, mainly from the viewpoint of the involvement of MN protein in oncogenesis; (2) determines if MN protein exerts carbonic anhydrase activity, and whether such activity is relevant for morphologic transformation of cells; and (3) tests whether MN protein is a cell adhesion molecule (CAM).
Synopsis
[0459] Methods: MN-cDNA was inserted into 3 expression vectors and was used for transfecting human or mouse cells. MN protein was detected by Western blotting, radioimmunoassay or immunoperoxidase staining; in all tests the MN-specific monoclonal antibody M75 (MAb M75) was used. Carbonic anhydrase activity was determined by the acidification velocity of carbonate buffer in CO2 atmosphere.
[0460] Results: (1) Cells (human CGL-1 and mouse NIH3T3 cells) transfected with MN-cDNA showed morphologi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| apparent molecular weight | aaaaa | aaaaa |
| apparent molecular weight | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com