Methods for cloning mammals using reprogrammed donor chromatin or donor cells
a technology of chromatin or donor cells, applied in the field of mammals cloning, can solve the problems of low efficiency of cloning mammals using donor somatic cells, loss of mid to late term pregnancies, and loss of nuclear transplant embryos, so as to increase the extent of reprogramming, improve the viability of cloned fetuses, and restore the membrane integrity of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Evidence For Nuclear Reprogramming Deficiencies in Traditional Bovine Nuclear Transplant Embryos
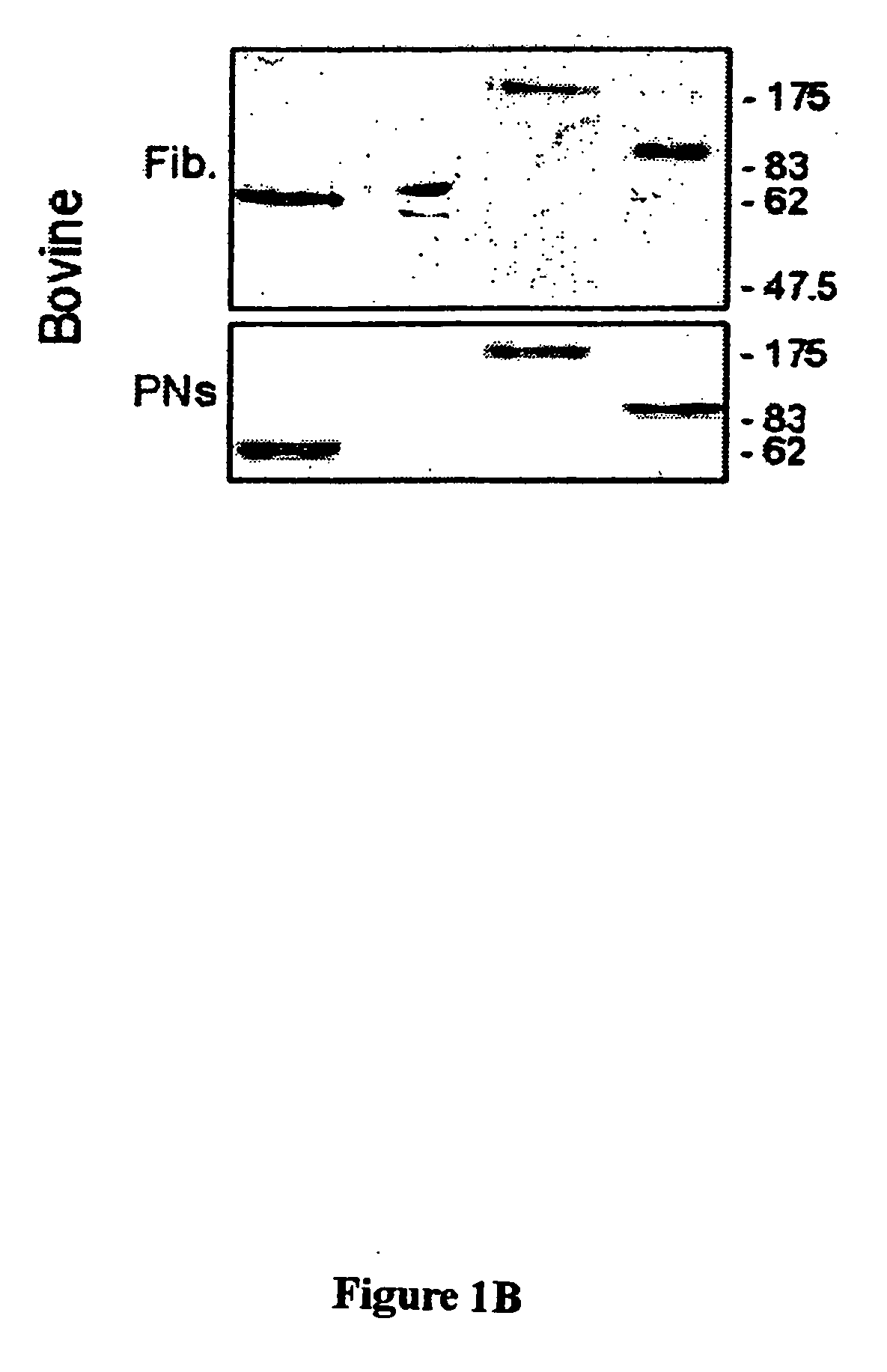
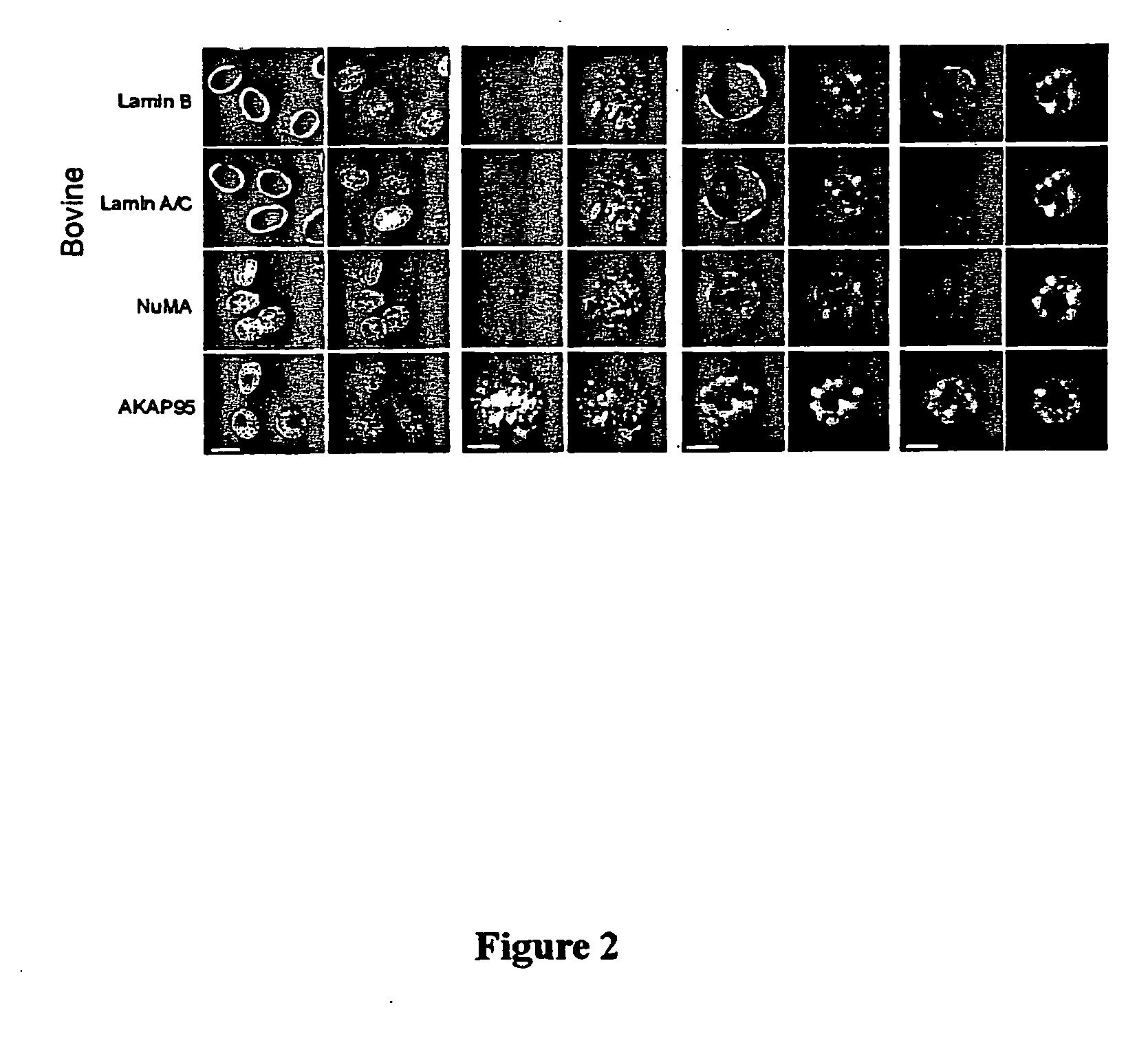
Distribution of Nuclear Envelope, Nuclear Matrix, and Chromatin-Matrix Interface Components During Bovine Preimplantation Development
[0073] To determine the distribution of nuclear envelope (B-type and A / C-type lamins), nuclear matrix (NuMA), and chromatin-matrix interface (AKAP95) components in preimplantation embryos, bovine embryos were produced by in vitro fertilization (IVF) and examined by immunofluorescence analysis. Bovine in vitro fertilization was performed as described previously (Collas et al., Mol. Reprod. Devel. 34:212-223, 1993). Briefly, frozen-thawed bovine sperm from a single bull was layered on top of a 45-90% Percoll gradient and centrifuged for 30 minutes at 700×g. The concentration of sperm in the pellet was determined, and the sperm was diluted such that the final concentration at fertilization was 106 sperm / ml. At 22 hours post maturation, oocytes were washed th...
example 2
Use of Reprogrammed Donor Chromatin Masses to Clone Mammals
[0088] To overcome the problem of incomplete reprogramming in traditional nuclear transfer embryos that was demonstrated in Example 1, new methods were developed to more efficiently reprogram donor chromatin prior to nuclear transfer. These methods involve incubating a nucleus from a donor cell in a reprogramming media (e.g., a cell extract) that results in nuclear envelope dissolution and possibly chromatin condensation. This nuclear envelope breakdown and chromatin condensation allows the release of transcription regulatory proteins that were attached to the chromosomes and that would otherwise promote the transcription of genes undesirable for oocyte, embryo, or fetus development. Additionally, regulatory proteins from the reprogramming media may bind the chromatin mass and promote the transcription of genes desirable for development.
Bulk Preparation of Donor Nuclei for Use in Cloning
[0089] As many as several million ...
example 3
Use of Reprogrammed Permeabilized Cells to Clone Mammals
[0115] Cells may also be reprogrammed without requiring the isolation of nuclei or chromatin masses from the cells. In this method, cells are permeabilized and then incubated in an interphase or mitotic reprogramming media under conditions that allow the exchange of factors between the media (e.g., a cell extract) and the cells. If an interphase media is used, the nuclei in the cells remain membrane-bounded; if a mitotic media is used, nuclear envelope breakdown and chromatin condensation may occur. After the nuclei are reprogrammed by incubation in this media, the plasma membrane is preferably resealed, forming an intact reprogrammed cell that contains desired factors from the media. If desired, the media can be enriched with additional nuclear factors as described in Example 2. The reprogrammed cells are then fused with recipient oocytes, and embryos formed from the reconstituted oocytes are inserted into maternal recipient ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com