Expression of xenogenous (human) immunoglobulins in cloned, transgenic ungulates
a technology of human immunoglobulin and ungulate, which is applied in the field of genetically modified ungulate, can solve the problems of insufficient human antibody availability for therapeutic and prophylactic use, demand exceeding supply, and routine shortfalls of human antibody availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Bovine IgM Knock Out
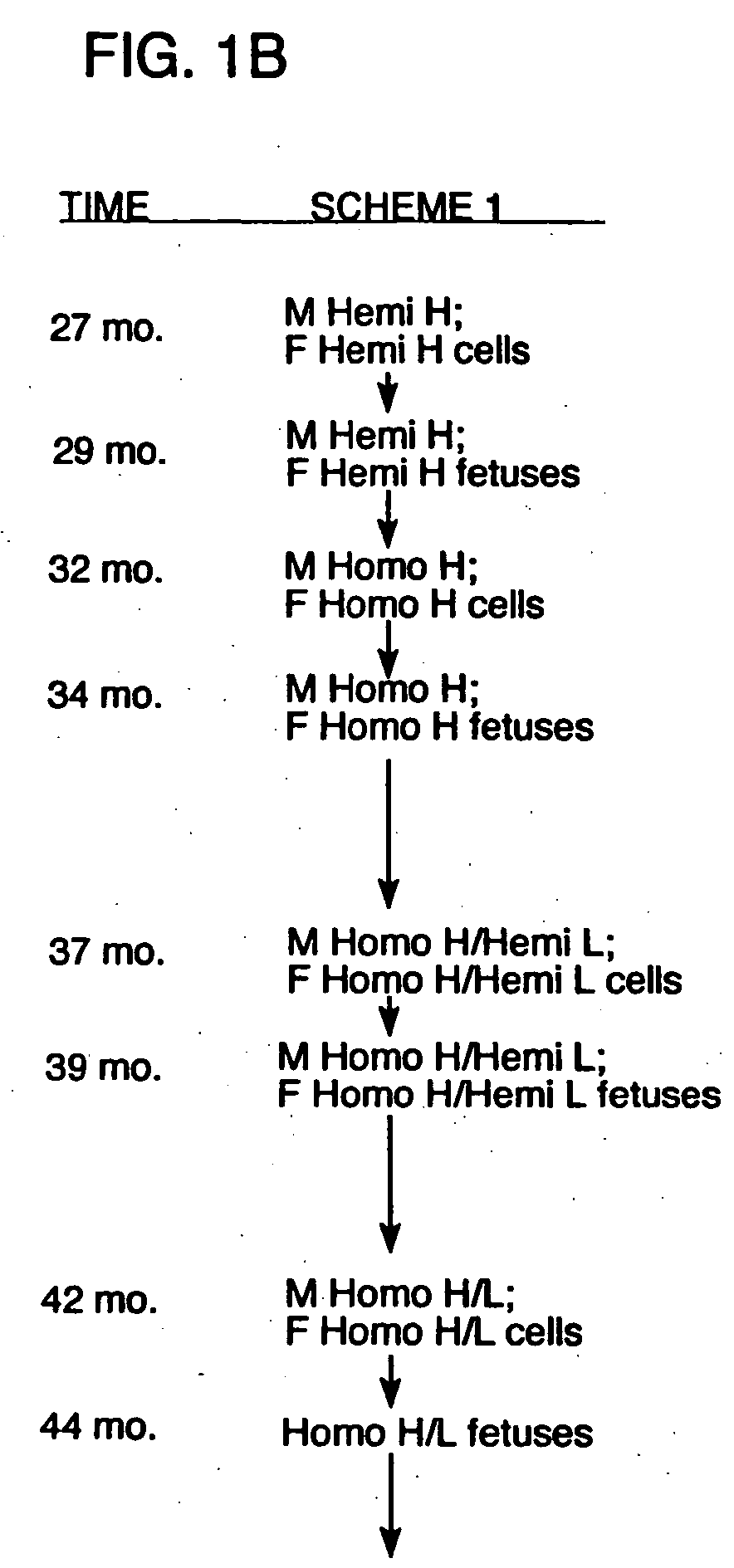
[0150] The following procedures were used to generate bovine fibroblast cell lines in which one allele of the immunoglobulin heavy chain (mu) locus is disrupted by homologous recombination. A DNA construct for effecting an IgM knockout was generated by the removal of exons 1-4 of the Mu locus (corresponds to IgM heavy chain gene) which were replaced with a copy of a neomycin resistance gene. Using this construct, neomycin resistant cell lines have been obtained which were successfully used in nuclear transfer procedures, and blastocysts from these cell lines have been implanted into recipient cows. Additionally, some of these blastocysts were tested to confirm that targeted insertion occurred appropriately in the mu locus using PCR procedures. Blastocysts resulting from nuclear transfer procedures from several of the cell lines obtained indicated that heterozygous IgM-KO fetuses were in gestation. Additionally, both male and female cell lines that comprise a sin...
example 2
Introduction of HAC
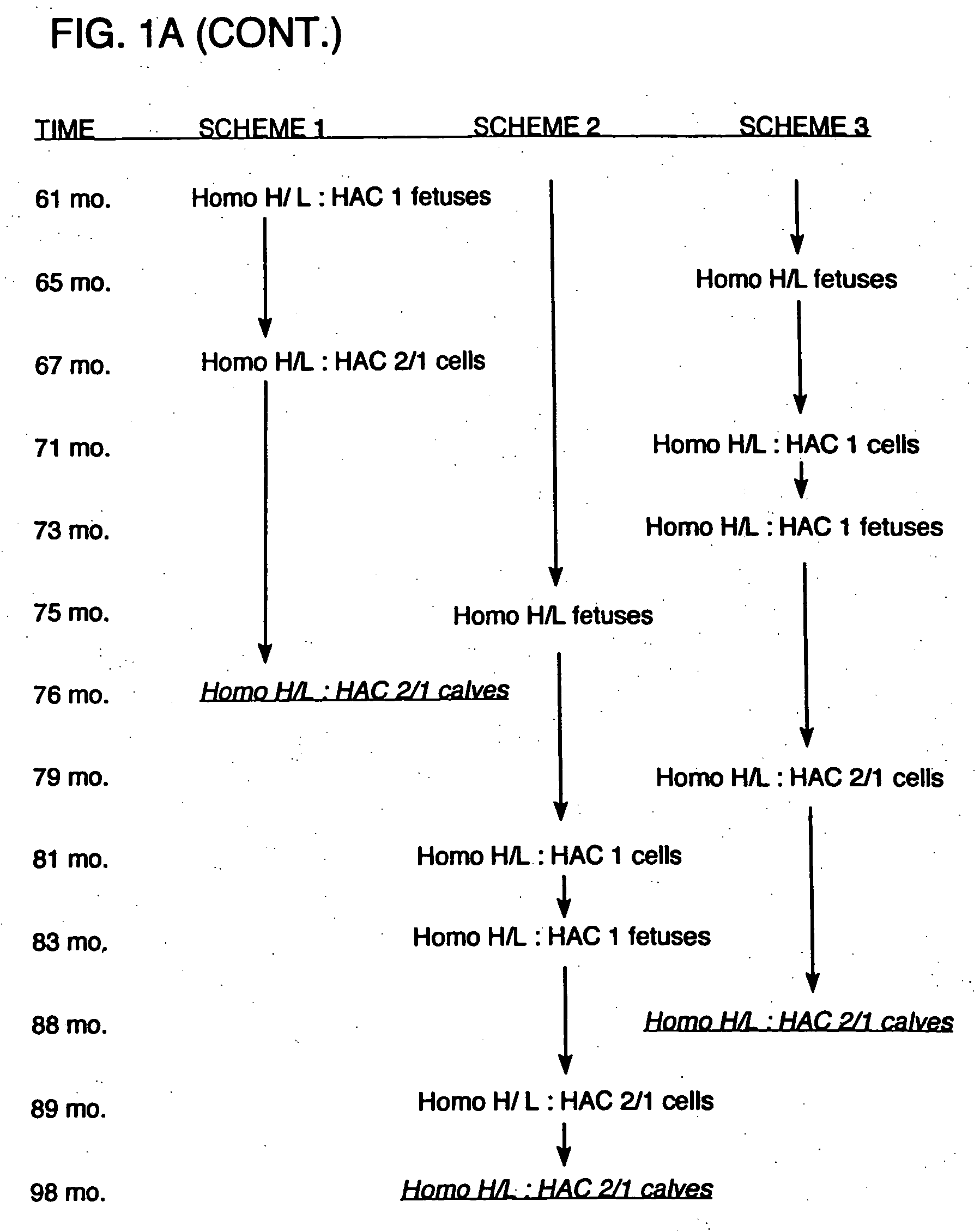
[0176] Additional experiments were carried out to demonstrate that immunoglobulin heavy chain (mu) and lambda light chain may be produced by a bovine host, either alone or in combination. In addition, these experiments demonstrated that the immunoglobulin chains were rearranged and that polyclonal sera was obtained. In these procedures, immunoglobulin-expressing genes were introduced into bovine fibroblasts using human artificial chromosomes. The fibroblasts were then utilized for nuclear transfer, and fetuses were obtained and analyzed for antibody production. These procedures and results are described in more detail below.
[0177] HAC Constructs The human artificial chromosomes (HACs) were constructed using a previously described chromosome-cloning system (Kuroiwa et al., Nature Biotech. 18: 1086-1090, 2000). Briefly, for the construction of ΔHAC, the previously reported human chromosome 22 fragment (hChr22) containing a loxP sequence integrated at the HCF2 locu...
example 3
Transgenic Ungulates Producing Xenogenous Antibodies and Reduced Amounts of Endogenous Antibodies
[0230] Transgenic ungulates expressing a xenogenous antibody and having a reduced level of expression of endogenous antibodies may also be generated. By increasing the number of functional xenogenous immunoglobulin heavy or light chain genes relative to the number of functional endogenous heavy or light chain genes, the percentage of B cells expressing xenogenous antibodies should increase.
[0231] To generate these transgenic ungulates, ΔHAC or ΔΔHAC transgenic ungulates may be mated with transgenic ungulates containing a mutation in one or both alleles of an endogenous immunoglobulin chain (e.g., a mu heavy chain or a lambda or kappa light chain). If desired, the resulting transgenic ungulates may be mated with (i) transgenic ungulates containing a mutation in one or both alleles of an endogenous alpha-(1,3)-galactosyltransferase, prion, and / or J chain nucleic acid or (ii) transgenic u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| total volume | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com