Methods of treating tumors using natural killer cell lines
a technology of natural killer cells and tumors, applied in the field of natural killer cells, can solve the problems of difficult immunotherapy work, difficult to work with and apply, and high treatment cost, and achieve the effects of promoting cell growth, reducing tumor cytotoxicity, and promoting cytokine secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
NK-92 Cells
[0083] NK-92 cells (Gong et al. (1994)) were derived from cells obtained from a patient suffering from non-Hodgkin's lymphoma. PBMC from the patient were cultured in enriched alpha MEM supplemented with fetal calf serum (12.5%) and horse serum (12.5%) plus 10−6 M hydrocortisone and 1000 U / mL of recombinant human IL-2 (rhIL-2). Cells were cultured at 37° C. in humidified air containing 5% CO2. Subcultures were made after 4 weeks, and propagated indefinitely with twice-weekly changes in medium. In these later stages the hydrocortisone could be omitted without any effect on cell growth. This culture has been designated NK-92 and has been deposited with the American Type Culture Collection (ATCC; Rockville, Md.) under designation CLR-2407.
[0084] The cells have the morphology of large granular lymphocytes. The cells bear the CD56bright, CD2, CD7, CD11a, CD28, CD45, and CD54 surface markers. In contrast, they do not display the CD1, CD3, CD4, CD5, CD8, CD10, CD14, CD16, CD19,...
example 2
Cytotoxic Activity of NK-92 against Different Leukemic Cell Lines
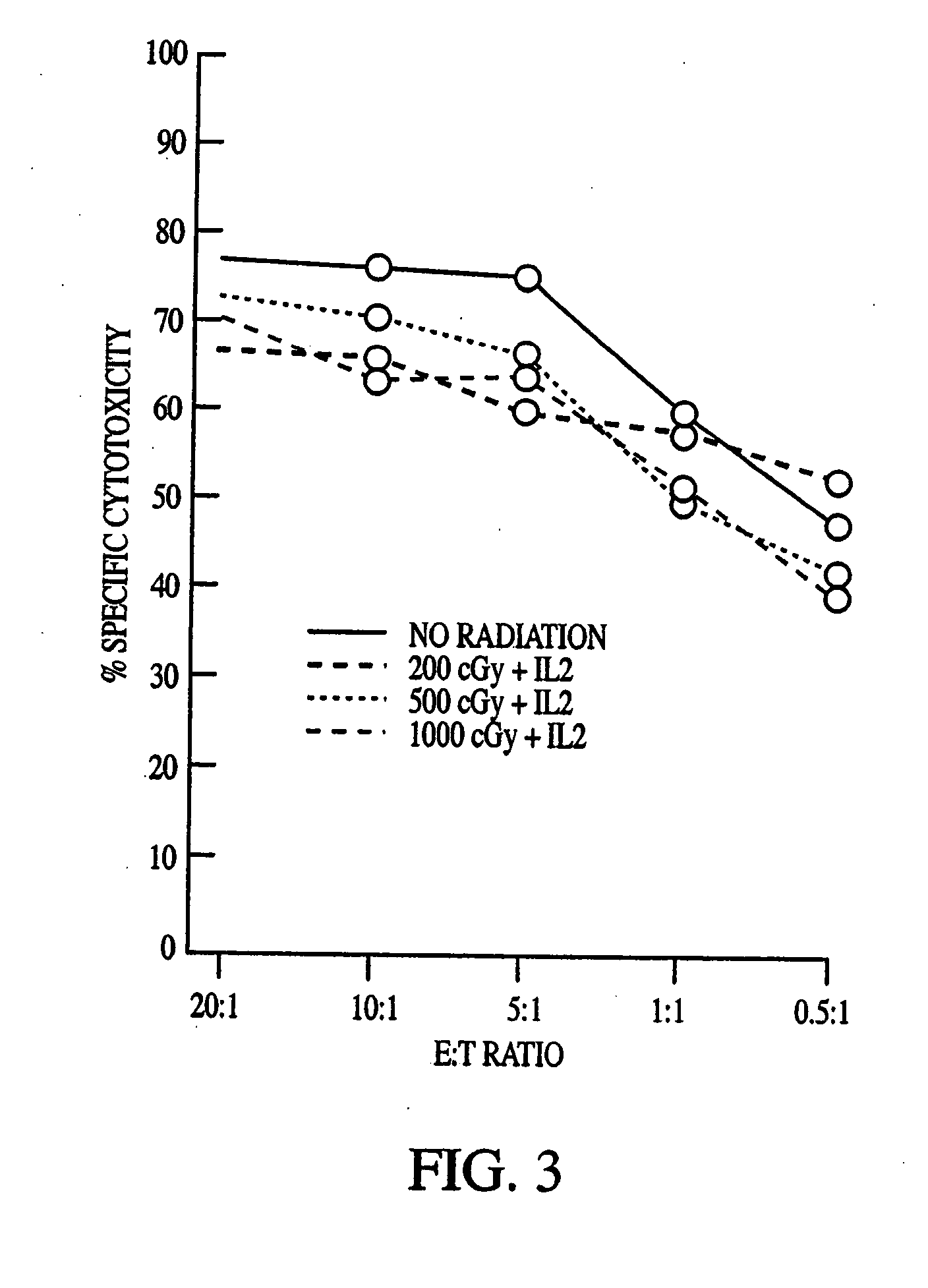
[0085] The cytotoxic activity of NK-92 against K562, Daudi, TF-1, AML-193, and SR-91 cells was determined (Gong et al. (1994)). K562 (erythroleukemia) and Daudi (Burkitt) lymphoma cell lines were obtained from ATCC. They were maintained in continuous suspension culture in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS). TF-1 is a myelomonocytic cell line (Kitamura et al., J. Cell Physiol. 140:323-334 (1989)) that requires the presence of medium containing 2 ng / ml of human GM-CSF. AML-193 is a myeloid cell line that is maintained in the presence of 10% 5637-conditioned medium (Lange et al., Blood 70:192-199 (1987)). Both TF-1 and AML-193 cells were obtained from Dr. D. Hogge, Terry Fox Laboratory, University of British Columbia, Vancouver, BC. SR-91 is a cell line with features of early progenitor cells established by Gong et al. (1994) from a patient with acute lymphoblastic leukemia (ALL) (Klingemann et...
example 3
Cytotoxicity of NK-92 against Leukemia, Lymphoma, and Myeloma Target Cell Lines
[0087] K562 (Ph-chromosome positive [Ph+] erythroleukemia), HL60 (promyelocytic). U937 (myelomonocytic), KG1a (variant subline of the AML cell line KG1), DHL-10 (B-cell lymphoma), Daudi (Burkitt's lymphoma), Raji (B-cell lymphoma), Jurkat (T-cell lymphoma), U266 (IgE myeloma), NCI H929 (IgA myeloma), and RPMI 8226 (myeloma, light chain secreting) cell lines were obtained from ATCC. The lymphoma-derived cell lines Ly3 (B-lineage, diffuse large cell), Ly8 (immunoblastic), and Ly13.2 (T-lineage, diffuse large cell) were provided by Dr. H. Messner, Toronto, Ontario. Their characteristics have been described (Chang et al., Leuk. Lymphoma 19:165 (1995)). All lines were maintained in RPM1 1640 medium supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 50 U / mL penicillin, 25 mM HEPES (StemCell Technologies), and 5% heat-inactive FCS (RPMI / 5% FCS) at 37° C. in a humidified atm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com