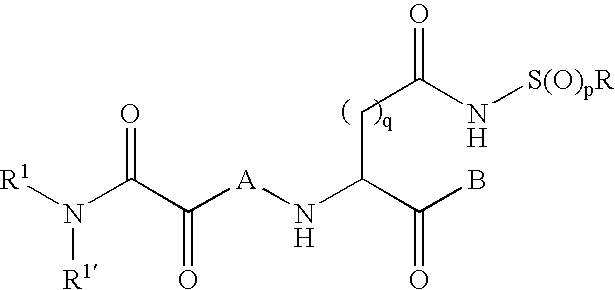
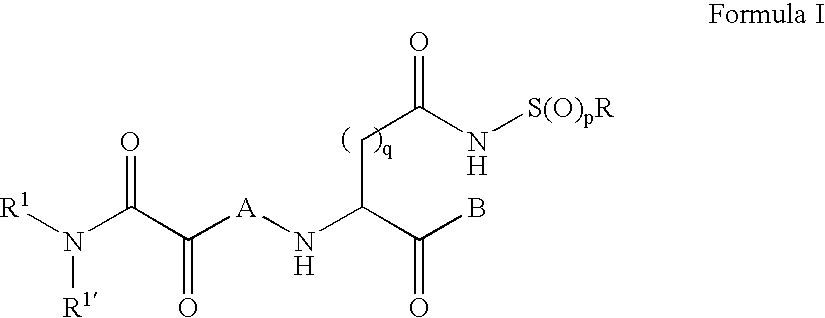
Inhibitors of the ICE/ced-3 family of cysteine proteases
a protease and inhibitor technology, applied in the field of inhibitors of the ice/ced3 family of cysteine proteases, can solve the problems of poor stability and rapid metabolism, unsatisfactory pharmacologic properties, poor oral absorption, etc., and achieve the effects of improving cell penetration, improving absorption and metabolic stability, and improving properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Assay for Inhibition of ICE / CED-3 Protease Family Activity
A. Determination of IC50 Values
[0155] Fluorescence enzyme assays detecting the activity of the compounds of Formula I utilizing the recombinant ICE and CPP32 enzymes are performed essentially according to Thomberry et al. (Nature 356:768:774 (1992)) and Nicholson et al. (Nature 376:37-43 (1995)) respectively, (herein incorporated by reference) in 96 well microtiter plates. The substrate is Acetyl-Tyr-Val-Ala-Asp-amino-4-methylcoumarin (AMC) for the ICE assay and Acetyl-Asp-Glu-Val-Asp-amino4-methylcoumarin for the CPP32, Mch2, Mch3 and Mch5 assays. Enzyme reactions are run in ICE buffer (25 mM HEPES, 1 mM EDTA, 0.1% CHAPS, 10% sucrose, pH 7.5) containing 2 mM DTT at room temperature in duplicate. The assays are performed by mixing the following components: [0156] 50 μL ICE, Mch2, Mch5, CPP32 (18.8, 38, 8.1 and 0.153 nM concentrations, respectively) or Mch3 (1 unit) enzyme in ICE buffer containing either 8.0 (ICE, Mch2, Mch...
example 2
3S)-N-methanesulfonyl-3-[N-(N′-(2-t-butylphenyl)oxamyl)valinyl]amino-5-(2′,3′,5′,6′-tetrafluorophenoxy)-4-oxapentanamide
[0161]
[0162] Compound No. 1 was made according to the following reaction scheme, the procedures for which are set forth below.
Bromomethylketone 2:
[0163] 4-Methylmorpholine (0.76 mL, 6.9 mmol) was added to a solution of Fmoc-Asp(OBn)-OH (1) (2.05 g, 4.62 mmol) in 50 mL of dry THF at −10° C. under an atmosphere of nitrogen, followed by the addition of isobutyl chloroformate (0.90 mL, 6.9 mmol), and the solution was stirred for 20 minutes. The resulting white precipitate was removed by filtration and the filtrate was cooled to 0° C. In a separate flask, 1-methyl-3-nitro-1-nitrosoguanidine (1.10 g, 7.44 mmol) was added to a vigorously stirred mixture of diethyl ether (14 mL) and 40% KOH (8 mL) at 0° C. The resulting mixture was stirred for 10 minutes and the layers were allowed to separate. The ether layer was transferred via plastic pipette to the mixed anhydride...
example 3
Representative Compounds
[0175] The representative compounds listed in the following Table I may be made according to the procedures set forth in Example 2.
TABLE 1Representative CompoundsCpdABR11NHCH(CH2CH(CH3)2)COH1-naphthyl2NHCH(CH2CH(CH3)2)COCH2F1-naphthyl3NHCH(CH(CH3)2)COCH2F1-naphthyl4NHCH(CH(CH3)2)COCH2OCO(2,4-diCl-Ph)1-naphthyl5NHCH(CH(CH3)2)COCH2O(2,6-diF-Ph)1-naphthyl6NHCH(CH(CH3)2)COCH2O(2,4,6-triF-Ph)1-naphthyl7NHCH(CH(CH3)2)COCH2O(2,3,5,6-tetraF-Ph)1-naphthyl8NHCH(CH(CH3)2)COCH2O(6-Me-2-pyron-4-yl)1-naphthyl9NHCH(CH(CH3)2)COCH2O(2-Ph-5,6-1-naphthylbenzopyran-4-on-3-yl)10NHCH(CH(CH3)2)COCH2OPO(Me)Ph1-naphthyl11NHCH(CH(CH3)2)COCH2OPOPh21-naphthyl12NHCH(CH(CH3)2)COCH2O(2-CF3-pyrimidin-4-1-naphthylyl)13NHCH(CH(CH3)2)COCH2O(5-CO2Me-isoxazol-1-naphthyl3-yl)14NHCH(CH(CH3)2)COCH2OPO(Me)(1-naphthyl)1-naphthyl15NHCH(CH2CH(CH3)2)COCH2OPOPh21-naphthyl16NHCH(CH2CH(CH3)2)COCH2OCO(2,6-diCl-Ph)1-naphthyl17NHCH(CH2CH(CH3)2)COCH2O(2,4,6-triF-Ph)1-naphthyl18NHCH(CH2CH(CH3)2)COCH2O(2,3,5,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| excitation wavelength | aaaaa | aaaaa |
| excitation wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com