Fusion protein of hiv regulatory/accessory proteins
a technology of hiv protein and fusion protein, which is applied in the field of fusion protein of hiv regulatory/accessory protein, can solve the problems of immune response, hiv protein production that is known to be harmful, and vaccine success not too well known
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Generation of a DNA Encoding a HIV Vif-Vpr-Vpu-Rev-Tat Fusion Protein
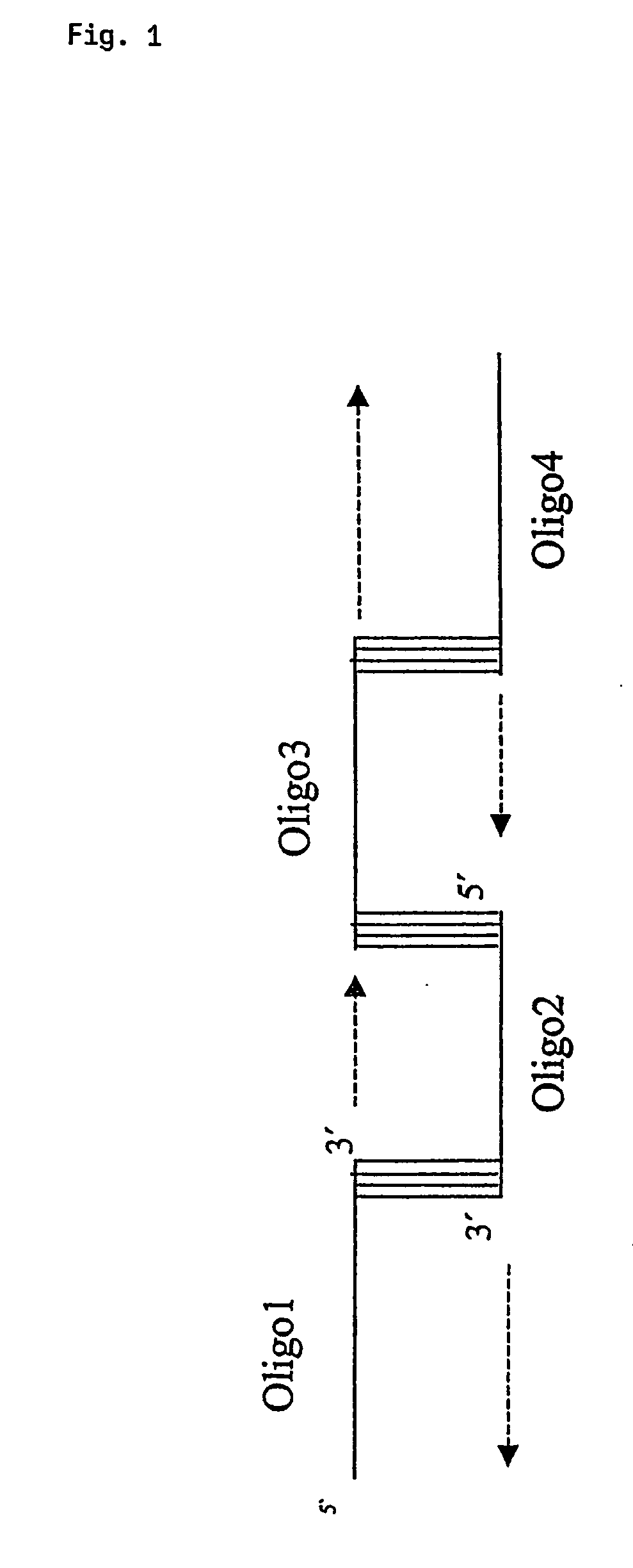
[0068] The single genes of the HIV genome were either prepared by PCR out of genomic DNA by using standard PCR protocols or synthetically by a technique, which is based on the annealing of oligonucleotides via overlapping sequences and fill in of the resulting single stranded gaps.
[0069] For the oligonucleotide based generation of coding regions of genes, which are to be inserted into the nucleic acid encoding the fusion protein according to the present invention, 40mer oligonucleotides with 15 bp overlaps were designed. The sequence of the oligonucleotides is based on the genomic map of the HIV1 isolate HXB2R that is derived from strain IIIB. The oligonucleotides for the annealing reaction or the PCR for isolation of the required sequence were designed in that way, that in the resulting coding region the stop codons for translation termination were deleted. The tat gene was synthesized using oligos containing a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com