Agonist antibodies
a technology of agonist antibodies and rtpo153, which is applied in the field of recombinant synthesis and purification of protein antibodies, can solve the problems of ineffective single doses of unglycosylated rtpo153, the inability to destroy essential stromal cells or components, and the inability to kill the body with radiation, etc., to achieve the effect of improving or modulating the half-li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0200] Assays
[0201] The mpl agonist antibody assays were conducted essentially as described in WO 95 / 18858.
[0202] (a) Ba / F3 Cell Proliferation Assay
[0203] The Ba / F3-mpl cell line was established (F. de Sauvage et al., Nature, 369:533 (1994)) by introduction of the cDNA encoding the entire mpl receptor into the IL-3 dependent murine lymphoblastoid cell line Ba / F3. Simulation of proliferation of Ba / F3-mpl cells in response to various concentrations of antibodies or TPO was measured by the amount of incorporation of 3H-thymidine as previously described (F. de Sauvage et al., supra).
[0204] (b) CMK Assay for Induction of Platelet Antigen GPIIbIIIa Expression
[0205] CMK cells are maintained in RMPI 1640 medium (Sigma) supplemented with 10% fetal bovine serum and 10 mM glutamine. In preparation for the assay, the cells are harvested, washed and resuspended at 5×105 cells / ml in serum-free GIF medium supplemented with 5 mg / l bovine insulin, 10 mg / l apo-transferrin, 1× trace elements. In ...
example 2
[0229] Isolation of Antibodies from the CAT Library
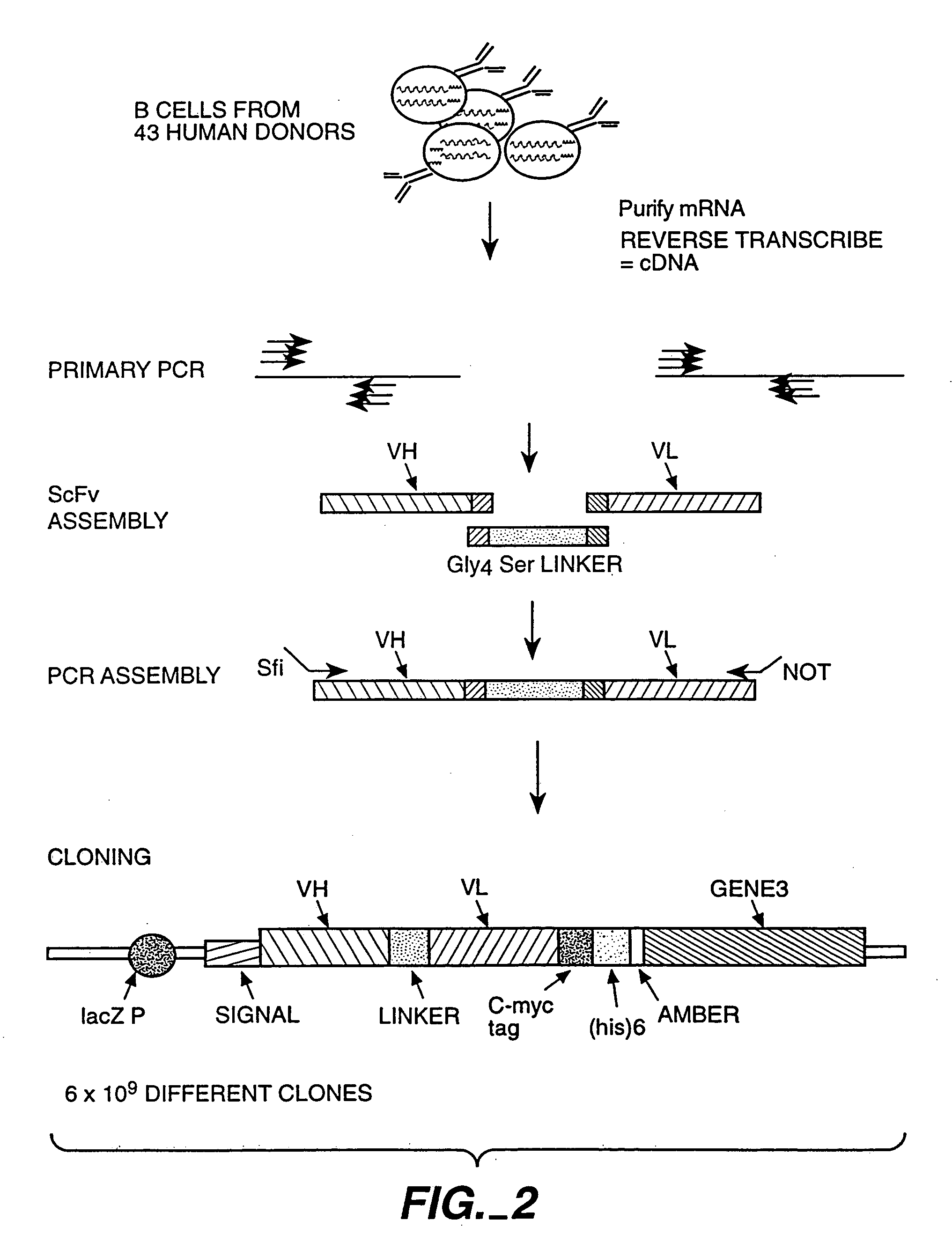
[0230] For construction of a library of antibodies displayed of a phage see the following references: WO 92 / 01047, WO 92 / 20791, WO 93 / 06213, WO 93 / 11236, WO 93 / 19172, WO 95 / 01438 and WO 95 / 15388. Briefly, FIGS. 2 and 3 presents a cartoon of the construction of a phage library containing 6×109 different clones containing single-chain Fv (scFv) antibodies fused to gene 3 of a phage. Binding selection against an antigen, in this case c-mpl, can be carried out as shown in FIG. 4 and described in greater detail below.
[0231] (a) The Antigen
[0232] Human c-mpl was cloned as described by F. de Sauvage et al., Nature 369:533 (1994).
[0233] (b) Phage Selection on Immunotubes
[0234] NUNC immunotubes were coated with 2 ml of a solution of 10 microg / ml of gD-c-mpl in PBS at 4° C. overnight. After rinsing with PBS, tubes were blocked with 3% dry milk in PBS (MPBS) for 2 hr at room temperature. For the first round, 10 μl of C.A.T. antibody phage...
example 3
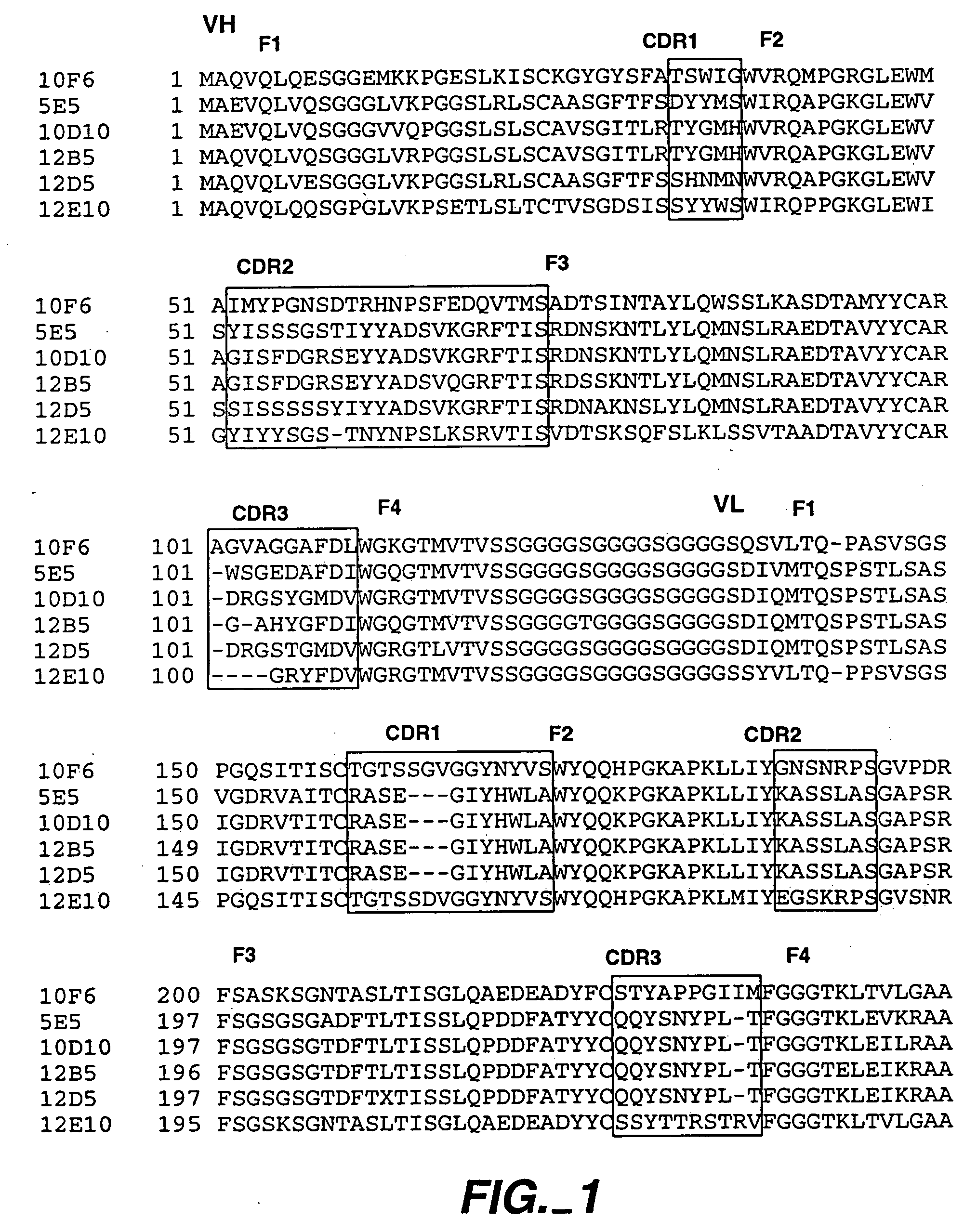
Cloning and Expression of Full Length Human Antibody Derivatives of 12B5, 12D5, and 12E10
[0291] For expression of full length antibodies in mammalian cells, the heavy chain variable domains were subcloned from the Fab constructs into a derivative of expression vector pRK (Suva et al., Science 237:893-896 (1987)) which contains the human IgG1 CH1, CH2, and CH3 domains and a human antibody signal sequence (Carter et al., Proc. Natl. Acad. Sci. USA. 89:4285-4289 (1992)). The light chain was cloned into a separate pRK plasmid. The light and heavy chain expression vectors were cotransfected into adenovirus-transformed human embryonic kidney cell line 293 by a high-efficiency procedure (Gorman et al, DNA Protein Eng. Technol. 2:3-10 (1990)). Harvested conditioned media was shown to contain anti-mpl antibody by ELISA.
[0292] For production of a more stable cell line and high-level antibody production, the light and heavy chains were moved into the SVI.DI expression vector previously descr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com