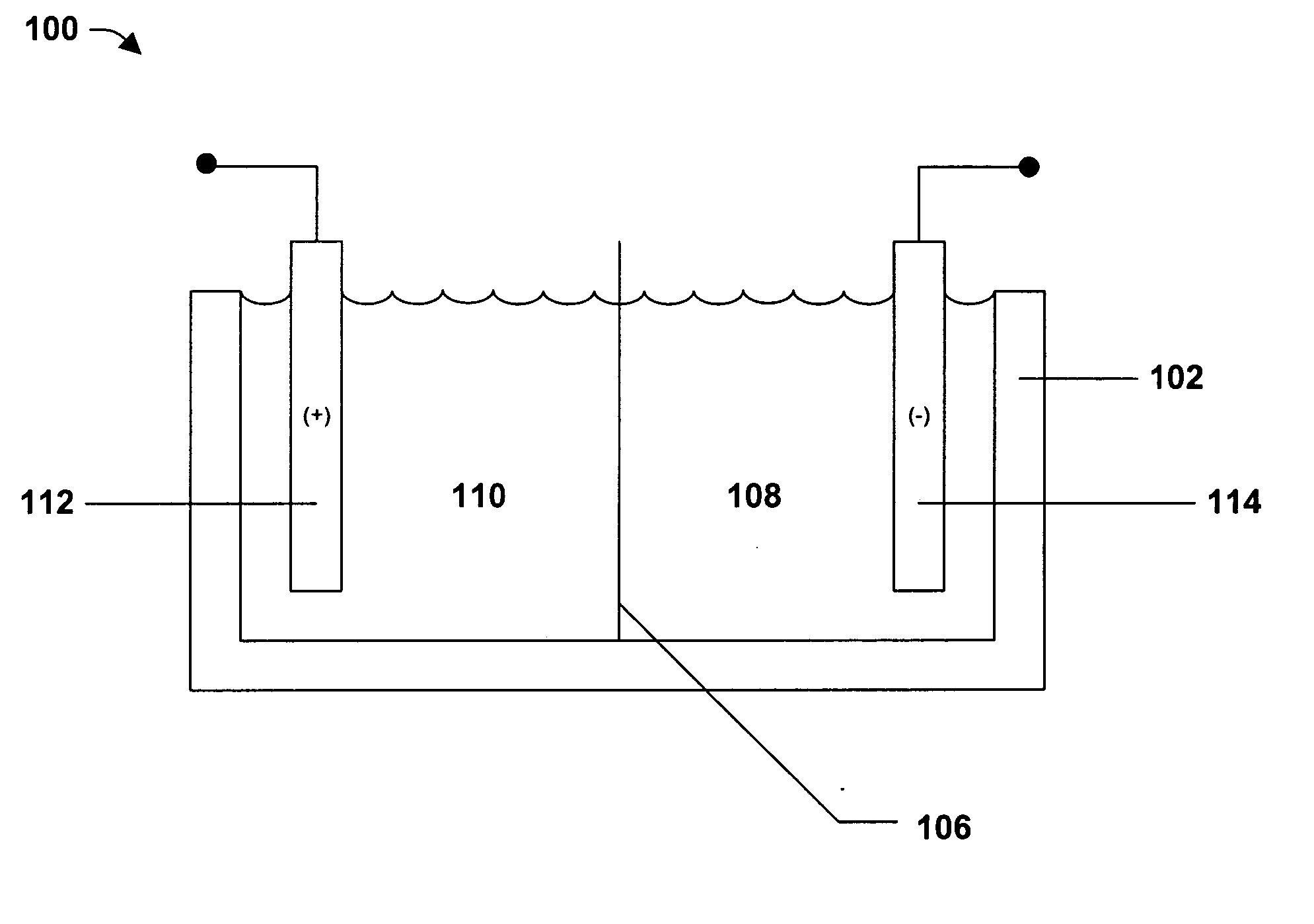
Nickel cobalt boron ternary alloys
a ternary alloy and cobalt technology, applied in the field of ternary alloys, can solve the problems of restricted use of chromium (vi) and its compounds in certain applications, and achieve the effects of increasing the bright plating range, and facilitating deposition of metal ions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0071] The electroplating bath of Comparative Example 1 is prepared except that 0.05 g / L of sodium laurel sulfate as a surfactant, 2.25 g / L of sodium saccharin as a stress reliever, 0.01 g / L of 2-butyne-1,4-diol and 0.01 g / L of propargyl alcohol as brighteners, and 0.10 g / L of 1-(sulfopropyl)pyridinium hydroxide as a leveler are added.
[0072] A metal deposit is produced from the electroplating bath with a surfactant, a stress reducing agent, and brighteners using 2 amp DC current in a mechanically agitated 267 ml Hull cell to determine plate response over a wide plating current density. The electroplating bath using a standard make up requires 6.2 Volts to achieve 2 amps. Plating time is 5 minutes. The type / appearance of the metal plate is shown in FIG. 4. A mirror bright deposit is obtained over at a wide range of current densities as low as about 4 ASF and higher.
example 2
[0073] The electroplating bath of Comparative Example 1 is prepared except that 0.1 g / L of sodium 2-ethylhexyl sulfate as a surfactant, 1 g / L of bisbenzenesulfonimide as a stress reliever, 0.01 g / L of 2-butyne-1,4-diol and 0.02 g / L of propargyl alcohol as brighteners, and 0.08 g / L of (1-sulfo-2-hydroxypropyl)pyridinium hydroxide as a leveler are added.
[0074] A metal deposit is produced from the electroplating bath with a surfactant, a stress reducing agent, and brighteners using 2 amp DC current in a mechanically agitated 267 ml Hull cell to determine plate response over a wide plating current density. Plating time is 5 minutes. The type / appearance of the metal plate is shown in FIG. 4. A mirror bright deposit is obtained over at a wide range of current densities as low as about 4 ASF and higher.
example 3
[0075] The electroplating bath of Example 1 is prepared except that a 100 g / L of sodium sulfate decahydrate is added via the make up. Voltage is lowered to 5 Volts to achieve 2 amps without impacting the type / appearance of the metal plate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com