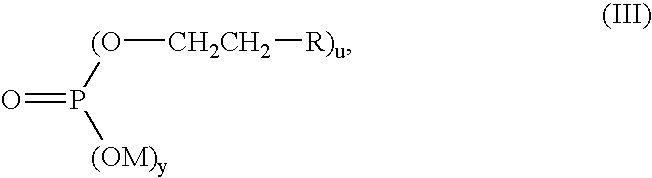Passivation composition and process for zinciferous and aluminiferous surfaces
a technology of aluminiferous and zinciferous surfaces, applied in the direction of solid-state diffusion coating, metal-based coating process, coating, etc., can solve the problems of surface staining and white corrosion, and achieve the effect of adequate corrosion resistan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Working compositions 1 and 2 were prepared as set forth below in Table 1.
TABLE 1Passivate CompositionsComposition 1Composition 2(pH ˜1.7)(pH ˜1.7)Ingredientwt. (g)wt. (g)DI Water92.577.575% H3PO43.54.050% H2TiF64.03.5TD-1355-DE—15.0(10% solids)
Test panels of HDG (hot dipped galvanized) steel and Galvalume® steel were prepared in the following manner.
The HDG steel panels were cleaned with a 3 wt. % solution of Parco® Cleaner 1200, available from Henkel Corporation, at a temperature of about 140° F. for about 20 seconds. The panels were then hot water rinsed at a temperature of about 120° F. for about 10 seconds. The panels were then squeegeed dry. Compositions 1 and 2 were then diluted to about 66 wt. % and were then applied to the panels using a No. 3 draw bar. The panels were then dried in an IR oven. The total coating weight on the panels were about 4-8 mg / ft2.
Galvalume® steel panels were cleaned and treated in the same manner except they were only cleaned for about 7 seco...
example 2
Working compositions 3 and 4 were prepared as set forth below in Table 4.
TABLE 4Passivate CompositionsComposition 3Composition 4(pH ˜1.7)(pH ˜1.7)Ingredientwt. (g)wt. (g)DI Water945.090.575% H3PO425.04.050% H2TiF630.03.5TD-1355-DE—2.0(10% solids)
HDG and Galvalume® steel test panels were prepared and subjected to NSS testing for 336 hours in the manner set forth above in Example 1. The results are shown below in Table 5.
TABLE 5NEUTRAL SALT SPRAY TESTING RESULTS ON GALVALUME AND HDG:% WHITE RUSTDayDayDayDayDayDayDayDay123456714Panel#CompositionSubstrateApplicationAppearance24487296120144168336133Galvalume ®#3 dbSplotchy1133103333100144-6 mg / ft21131033333310015Coating wt.001310333350163Galvalume ®coaterPerfect3100100100100100100100172-4 mg / ft2310010010010010010010018Coating wt.3100100100100100100100284Galvalume ®#3 dbPerfect0000000.11294-8 mg / ft200000.10.10.1130Coating wt.0000000.131101Galvalume ®#3 dbPerfect000135101001114-8 mg / ft200115510100112Coating wt.Dark01155531001132Galva...
example 3
HDG and Galvalume® steel test panels were cleaned with a 4 wt. % solution of Ridoline® 321 at a temperature of about 140° F. for about 30 seconds. The panels were then hot water rinsed at a temperature of about 120° F. for about 10 seconds. The panels were then squeegeed dry. Composition 1 was applied to the panels using various drawbars. The test panels were then subjected to (i) Stack testing, (ii) Butler Water Immersion testing, and (iii) NSS testing for 72 hours in the manner set forth above in Example 1. The results are shown below in Tables 6, 7, and 8.
TABLE 6Stack Test Results(% Black Rust (BR) White Rust (WR))Substrate / Total Coat.CoatingWt.168 Hrs336 Hrs672 Hrsmethod(mg / ft2)BRWRBRWRBRWRHDG8-1200110333(#9 drawbar)Galvalume4-8 001 13 1(#3 drawbar)
TABLE 7Butler Water Immersion Test Results(% Black Rust (BR) White Rust (WR))Substrate / Total Coat.CoatingWt.168 Hrs336 Hrs672 Hrsmethod(mg / ft2)BRWRBRWRBRWRHDG8-12111033333(#9 drawbar)Galvalume4-8 11 3333 3(#3 drawbar)
TABLE 8Neutral...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com