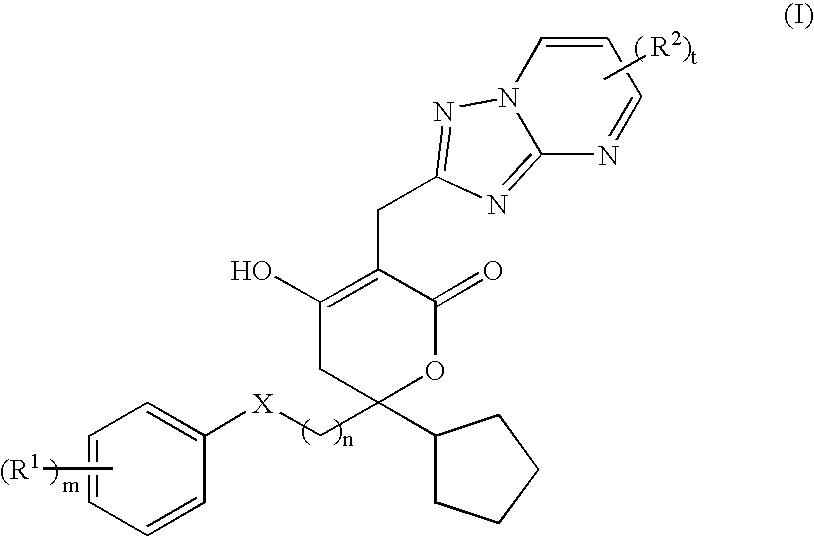
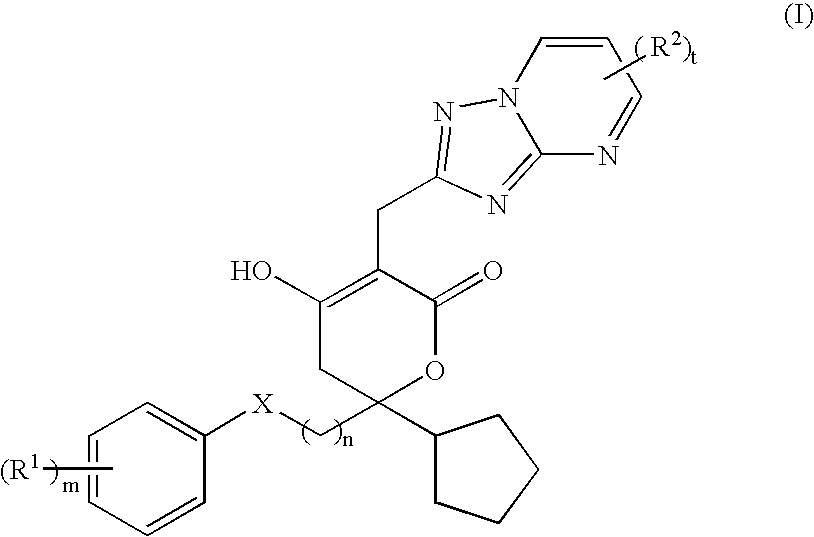
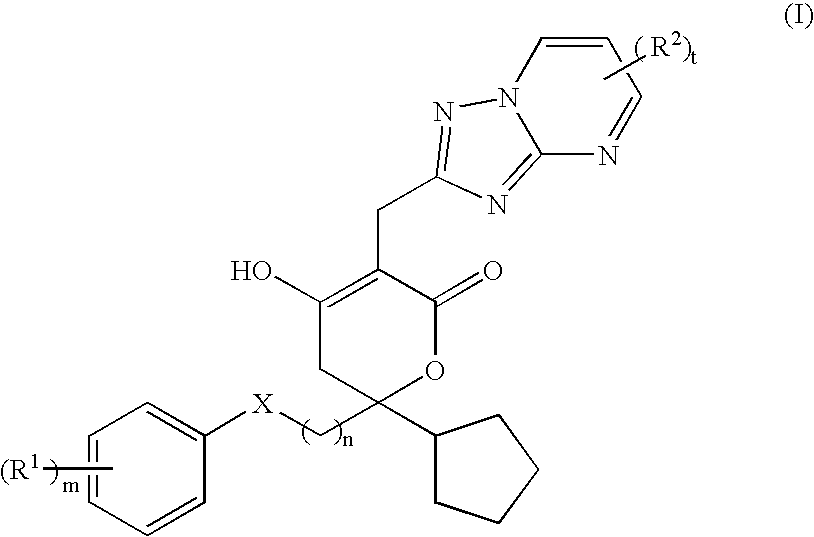
Pharmaceutical compositions and methods for their use
a technology of compositions and pharmaceuticals, applied in the field of pharmaceutical compositions, can solve the problems of not producing the desired physiological effect, requiring more frequent and higher doses of drugs, etc., and achieve the effect of increasing the bioavailability of mammal cells and increasing the bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
6-Cyclopentyl-3-(5,7-dimethyl-[1,2,4]triazolo[1,5-a]pyrimidin-2-ylmethyl)--6-(2-(3-ethyl-4-hydroxy-phenyl)-ethyl]-4-hydroxy-5,6-dihydro-pyran-2-one
[0206] 21
[0207] The desired product was prepared analogously to example 1, substituting 6-Cyclopentyl-6-[2-(3-ethyl-4-hydroxy-phenyl)-ethyl]-dihydro--pyran-2,4-dione. from Step 4 below, in place of 2-{4-[2-(2-cyclopentyl-4,-6-dioxo-tetrahydro-pyran-2-yl)-ethyl]-2-fluoro-phenyl}-2-methyl-propionitr-ile of that example. .sup.1H NMR (DMSO-d.sub.6): .delta. 0.96 (t, 3H, J=7.4 Hz), 1.30-1.58 (br m, 8H), 1.95 (m, 2H), 2.41 (m, 12H), 2.63 (d,1 H, J =17.5 Hz), 3.61 (d, 1H, J=15.8 Hz), 3.72 (d, 1H, J=15.8 Hz), 6.52 (d, 1H, J=8.1 Hz), 6.74 (m, 2H), 6.93 (s, 1H), 8.84 (s,1 H). Anal. Calcd. For C.sub.28H.sub.34N.sub.4O.sub.4.0.5 AcOH: C, 66.90; H, 6.97; N, 10.76. Found: C, 66.89; H, 6.97, N, 10.83.
Step 1: 4-Bromo-2-ethyl-phenol
[0208] 22
[0209] Sodium hydroxide (1.4 g, 35 mmol) and hydrazine monohydrate (2.04 mL, 42 mmol) were added to a solution of 5'...
example 3
1-(4-{2-[2-Cyclopentyl-5-(5,7-dimethyl-[1,2,4]triazolo[1,5-a]pyrimidin-2-y-lmethyl)-4-hydroxy-6-oxo-3,6-dihydro-2H-pyran-2-yl]-ethyl}-2-fluoro-phenyl-)-cyclopropanecarbonitrile
[0217] 26
[0218] The desired product was prepared analogously to example 1, substituting 1-{4-[2-(2-Cyclopentyl-4,6-dioxo-tetrahydro-pyran-2-yl)-ethy-l]-2-fluoro-phenyl}-cyclopropanecarbonitrile (0.24 g, 0.65 mmol) from Step 3 below, in place of 2-{4-[2-(2-cyclopentyl-4,6-dioxo-tetrahydro-pyran-2--yl)-ethyl]-2-fluoro-phenyl}-2-methyl-propionitrile of that example. Yield: 64 mg, 19%. .sup.1H NMR (CDCl.sub.3) .delta.: 1.25-1.30 (m, 2H), 1.42-1.68 (m, 10H), 1.88-1.93 (m, 2H), 2.30 (p, J=8.59 Hz, 1H), 2.44-2.73 (m, 10H), 4.05 (d, J=3.03 Hz, 2H), 6.76-6.84 (m, 3H), 7.09-7.22 (m, 1H).
Step 1: 1-(4-Bromo-2-fluoro-phenyl)-cyclopropanecarbonitrile
[0219] 27
[0220] To a slurry of sodium hydride (60% dispersion in mineral oil, 0.82 g, 20.6 mmol) in DMF (20 mL) cooled to 0.degree. C. was added a solution of (4-bromo-2-fluoro-...
example 4
2-[4-(2-{2-cyclopentyl-4-hydroxy-5-[(6-methyl[1,2,4]triazolo[1,5-a]pyrimid-in-2-yl)methyl]-6-oxo-3,6-dihydro-2H-pyran-2-yl}ethyl)-2-fluorophenyl]-2-m-ethylpropanenitrile
[0225] 30
[0226] The title compound was prepared analogously to Example 1 where 6-Methyl-[1,2,4]triazolo[1,5-a]pyrimidine-2-carbaldehyde from step 2 below was substituted in place of 5,7-dimethyl-[1,2,4]triazolo[1,5-a]pyri-midine-2-carbaldehyde in the final step of that example. .sup.1H NMR (400 MHz, CDCl.sub.3) .delta.: 1.48-1.71 (m, 8H), 1.76 (s, 6H), 2.00 (m, 2H), 2.06 (s, 1H), 2.10 (s, 1H), 2.38 (m, 1H), 2.39 (s, 3H), 2.68 (m, 2H), 2.81(m, 1H), 4.09 (s, 2H), 6.86 (d, J=12.9 Hz, 1H), 6.92 (d, J=8.1 Hz, 1H), 7.84 (t, J=8.1 Hz, 1H), 8.63 (s, 1H), 8.70 (s, 1H). MS (ESI): 518.6(M+H.sup.+).
Step 1: Preparation of (6-methyl[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)metha-nol
[0227] 31
[0228] To a solution of (3-amino-1H-1,2,4-triazol-5-yl)methanol (16.6 g, 87.6 mmol) in acetic acid was added 3-ethoxymethacrolein (10.0 g, 87.6 mmo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com