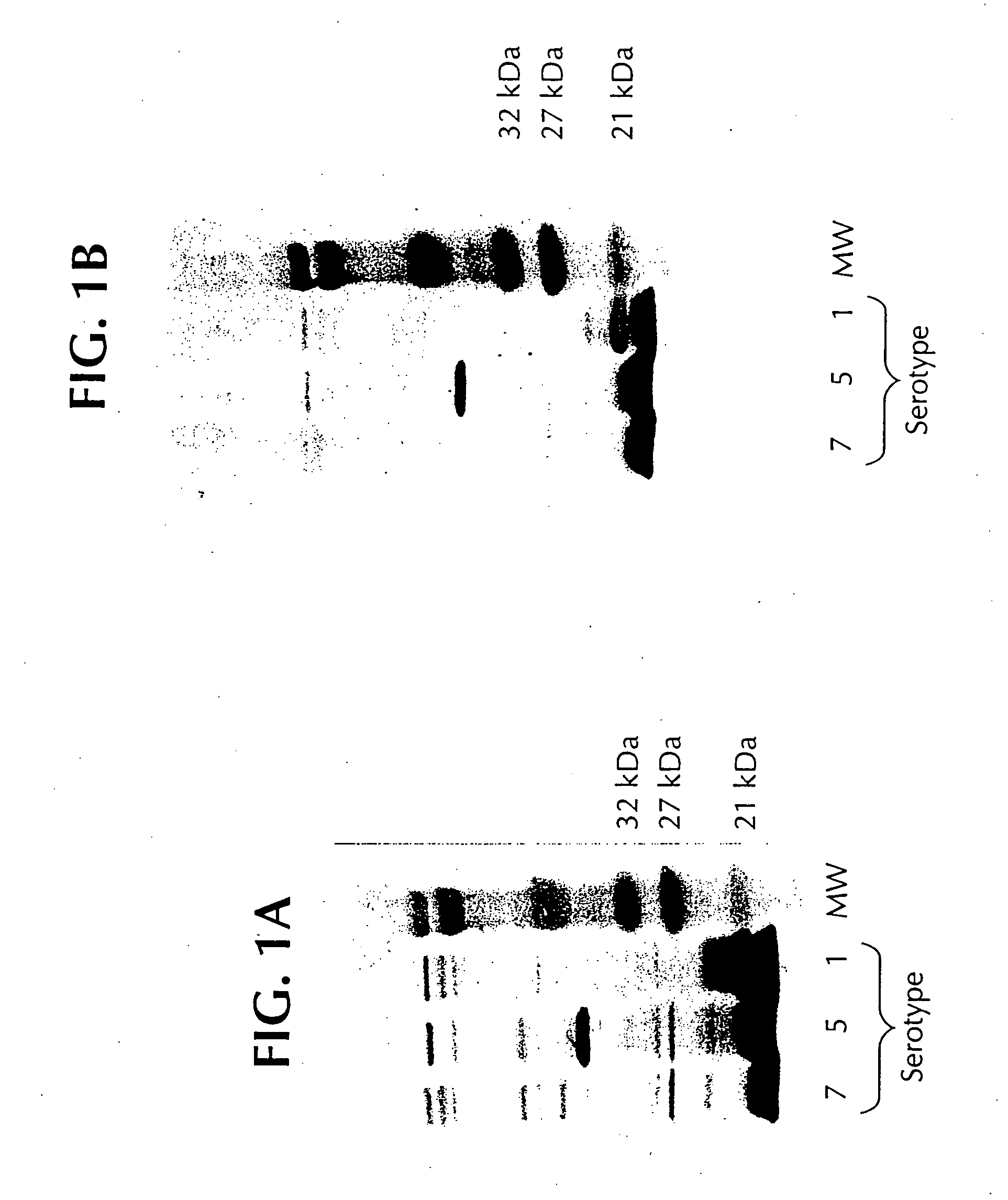
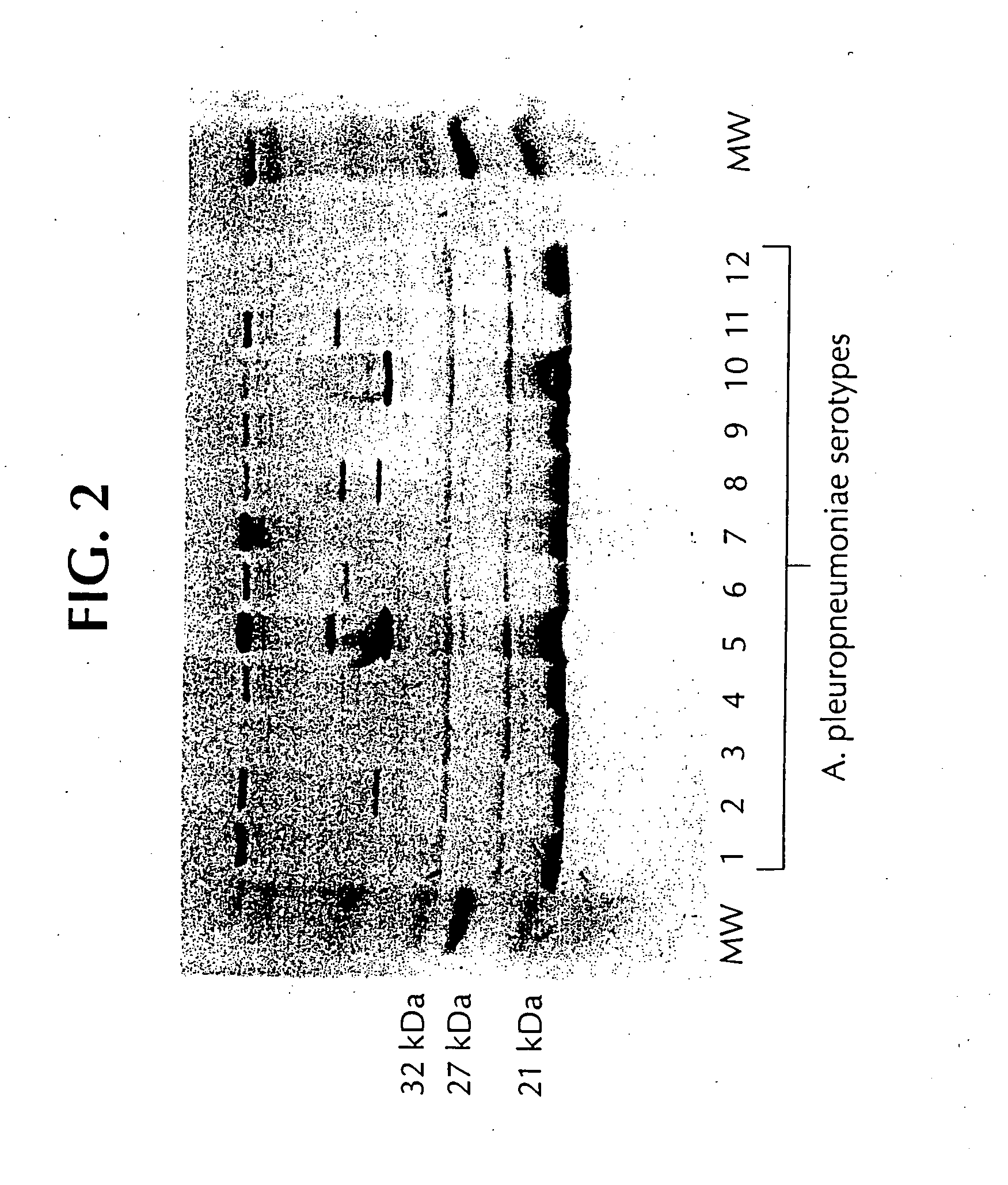
Novel proteins from Actinobacillus pleuropneumoniae
a technology of actinobacteria and proteins, applied in the field of animal health, can solve the problems of limited cross-protective response induced by natural infection, inability to identify possible differences between the specificity of antibodies during primary versus secondary responses, and inability to isolate individual components or combinations of individual components from apps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 10
Animal Study to Test Efficacy of Various Antigen Combinations
10.1 Materials and Methods
[0218] Fifty apparently healthy, crossbred pigs (approximately 6.5 weeks of age) were obtained from a herd with no history of APP disease or vaccination. Animals were randomly assigned by litter and by ApxII cytolytic neutralization antibody titer to five groups of 10 pigs (98% of the animals had serum neutralization titers <1:200 prior to the initiation of study). Pigs were acclimated for one week prior to initiation of study.
[0219] Animals were vaccinated with 2 ml of the appropriate vaccine (APP proteins with pp and without signal sequence) or placebo by the intramuscular route (IM; left neck muscle) on day 0, when pigs were approximately 7.5 weeks of age. After 3 weeks, animals were boosted with a second 2 ml dose (IM; right neck muscle). TABLE 6 identifies the vaccines used for first and second vaccinations of the 5 groups of pigs.
6TABLE 6 Vaccine Group Vaccine Components A 75 .mu.g pp-OmpW 7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weights | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com