Vaccine to protect a pig against actinobacillus pleuropneumoniae
一种胸膜肺炎、放线杆菌的技术,应用在针对胸膜肺炎放线杆菌保护猪的疫苗领域,能够解决没有显示出猪保护作用等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the recombinant expression of ApxI
[0023] Construction of transfer vector pFastbac-ApxIA
[0024] The rApxIA gene was synthesized based on Actinobacillus pleuropneumoniae strain 4074 (Swissprot accession number: P55128). The gene was codon-optimized for baculovirus polyhedrin usage and included a Kozak sequence (TATAAAT) and a 3' hexahistidine tag. The rApxIA-His gene was cloned behind the polyhedrin promoter of plasmid pFastbac1 (Life Technologies, Carlsbad, USA) as a BamHI fragment to obtain plasmid pFastbac-ApxIA TAT.
[0025] Production of Recombinant Baculovirus BacdCCApxIA-TAT
[0026] Recombinant baculoviruses were produced in the Bac to Bac system (Life Technologies, Carlsbad, USA) using the plasmids described above according to the manufacturer's protocol. The E. coli cells used for transformation contained the parental baculovirus and had chitinase and v-cathepsin gene deletions (Kaba SA, Salcedo AM, Wafula PO, Vlak JM, van Oers MM. J Virol ...
Embodiment 2
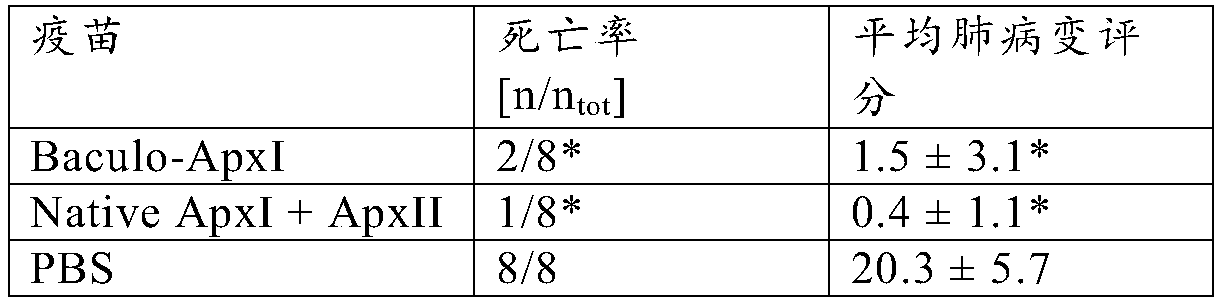
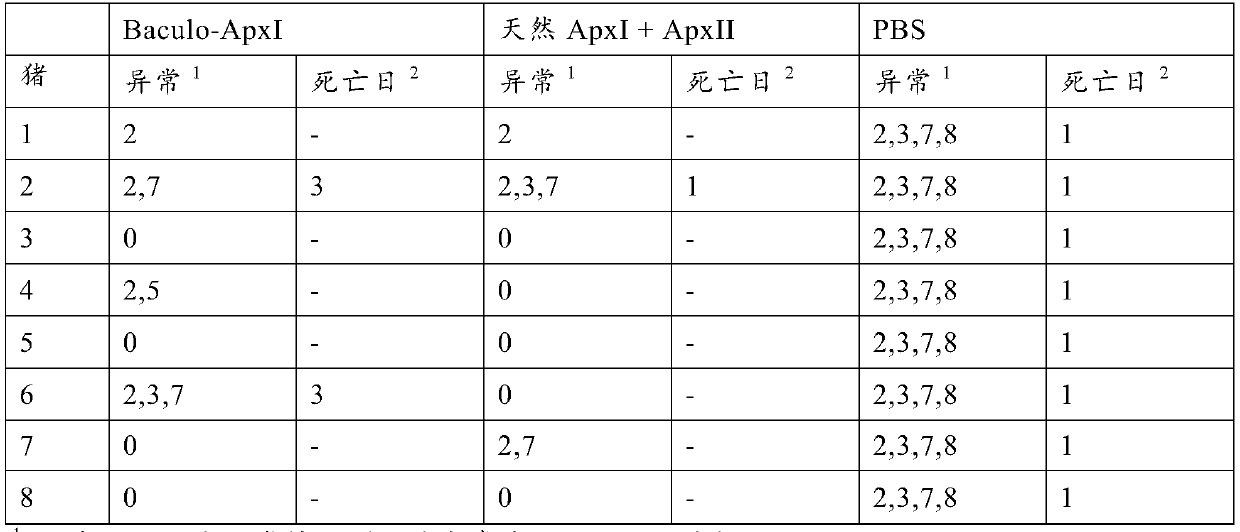
[0030] Example 2: Vaccine Effectiveness
[0031] Vaccine preparation
[0032] Two different vaccines were prepared for the study. The first vaccine comprises purified baculovirus expressing ApxI as obtained using the method described in Example 1. A second vaccine versus a commercially available vaccine as a positive control APP is comparable, comprising ApxI and ApxII purified from the culture supernatant of A. pleuropneumoniae (and thus complexed with LPS), also known as "native ApxI+ApxII". Investigational vaccines differ from commercially available vaccines APP because it does not contain the ApxIII toxin. However, this was irrelevant for challenge with serotype 10 field strains (serotype 10 does not produce ApxIII). Antigens were mixed with mineral oil-containing adjuvant (XSolve, available from MSD AnimalHealth, Boxmeer, The Netherlands) at a final concentration of 25 μg / ml of each antigen.
[0033] vaccination program
[0034] 3 groups of 8 piglets from an A. p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com