Benzoxazepine compounds, their production and use
a technology of benzoxazepine and compound, applied in the direction of biocide, drug composition, cardiovascular disorder, etc., can solve the problem of feared undesirable side effects and achieve the effect of excellent lipid-level lowering activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
working example 1
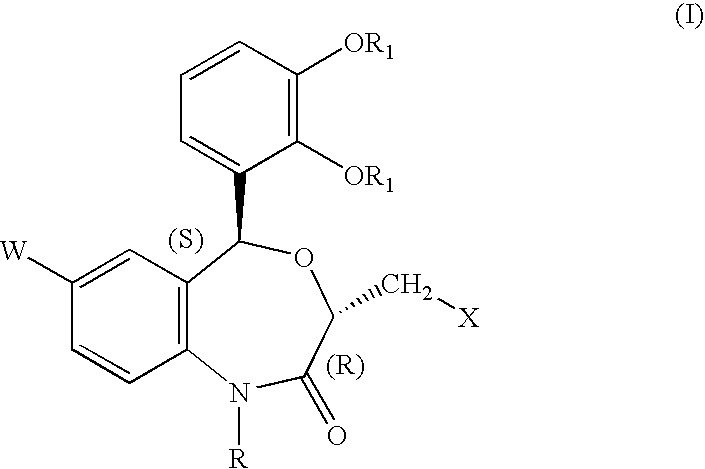
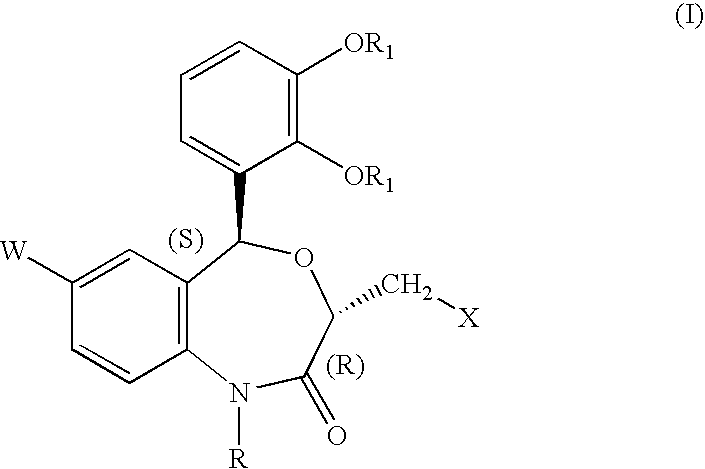
[0248] Methyl ester of N-[(3R,5S)-7-chloro-5-(2,3-dimethoxyphenyl)-1-neope-ntyl-2-oxo-1,2,3,5-tetrahydro-4,1-benzoxazepine-3-acetyl]piperidine-4-carb-oxylic acid 16
[0249] To a solution of (3R,5S)-7-chloro-5-(2,3-dim thoxyphenyl)-1-n opentyl-2-oxo-1,2,3,5-tetrahydro-4,1-benzoxazepin -3-acetic acid (0.5 g) and 0.25 g of piperidine-4-carboxylic acid methyl ester hydrochloride in dimethylformamide (10 ml) were added, at room temperature, diethylcyanophosphonate (0.28 g) and triethylamine (0.38 ml), and the mixture was stirred for one hour. To the mixture were added water (100 ml) and ethyl acetate (100 ml). The organic layer was washed with 1N HCl and a saturated aqueous solution of sodium hydrogencarbonate, followed by drying over an hydrous magnesium sulfate. The solvent was distilled off, and the residue was purified by silica-gel column chromatography (eluents: hexane: ethyl acetate =1:1 (v / v) to afford 0.62 g of a colorless crystalline product, m.p. 124-126.degree. C.
[0250] Element...
working example 2
[0253] By substantially the same procedure as in Example 1, compounds shown in [Table 1] were obtained.
1TABLE 1 17 Compound No. Y m.p. (.degree. C.) 2-1 18 159-160 2-2 19 110-112 2-3 20 200-202 2-4 21 123-125 2-5 22 196-198 2-6 23 169-171 2-7 24 256-258 2-8 25 175-177 2-9 26 86-89 2-10 27 154-155 2-11 28 141-142 2-12 29 146-148 2-13 30 111-113 2-14 31 125-127 2-15 32 180-180.5 2-16 33 195-197 2-17 34 203-204 2-18 35 132-134 2-19 36 197-200 2-20 37 165-166 2-21 38 142-145 2-22 39 209-210 2-23 40 123-125 2-24 41 96-98 2-25 42 107-108 2-26 43 142-144 2-27 44 216-218 2-28 45 132-134 2-29 46 amorphous solid 2-30 47 amorphous solid 2-31 48 amorphous solid 2-32 49 104-106 2-33 50 115-116 2-34 51 103-105 2-35 52 193-195 2-36 53 126-128 2-37 54 124-127 2-38 55 150-151
working example 3
[0254] N-[(3R,5S)-7-chloro-5-(2,3-dimethoxyphenyl)-1-neopentyl-2-oxo-1,2,3-,5-tetrahydro-4,1-benzoxazepine-3-acetyl]piperidine-4-carboxylic acid 56
[0255] The compound (0.5 g) obtained in Example 1 was dissolved in a mixture of 1N aqueous solution of sodium hydroxide (4 ml), methanol (10 ml) and tetrahydrofuran (5 ml). The solution was stirred for one hour at room temperature, to which were added 1N HCl (50 ml) and ethyl acetate (100 ml). The organic layer was washed with water and dried over anhydrous magnesium sulfate. The solvent was removed, and the residue was recrystallized from hexane-diethyl ether to afford 0.47 g of colorless crystals, m.p. 145-147.degree. C.
[0256] Elemental analysis for C.sub.30H.sub.37ClN.sub.2O.sub.7.0.3H.sub.2O-:
[0257] Calcd.: C, 62.29; H, 6.55; N, 4.84
[0258] Found: C, 62.20; H, 6.65; N, 4.83
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com